(2: 中国科学院大学, 北京 100049)
(2: University of Chinese Academy of Sciences, Beijing 100049, P. R. China)
湖泊沉积物是水体氮、磷等营养盐重要的汇,由于浅水湖泊易受风浪扰动作用发生再悬浮,造成沉积物氮、磷等营养物质释放到上覆水,因此比深水湖泊更易引起湖泊富营养化[1].目前太湖和巢湖等多个浅水湖泊面临水质恶化和湖泊富营养化等问题[2-4].研究表明,磷(P)是湖泊蓝藻水华产生的限制性因子[5],内源P的释放是切断外源污染后, 富营养化现象持续存在的重要原因[6-8].
在探讨内源P释放机制时,铁、铝、钙结合态磷(Ca-P、Al-P、Fe-P)等都纳入研究范围,其中Fe-P是沉积物中最易受环境影响而引起磷释放的形态[9].目前,大量研究围绕沉积物Fe和P的关系展开[10-12]. Bortleson等[13]研究发现湖泊沉积物中P和Fe呈现显著的相关性.进一步的研究表明,Fe含量高的沉积物,NH4Cl-P、NaOH-P浓度相对也高[14]. Søndergaard等[15]对丹麦浅水湖泊沉积物进行P形态分析时,提出沉积物表层P浓度受外源P负荷和Fe浓度影响,且随着两者浓度增加而增加.另外也有研究表明P的释放与P/Fe比值呈负相关,在P/Fe<1时,P的释放速度明显升高[16].这些发现都验证了Fe和P的循环对P的界面释放起重要作用,Fe3+的降低对P释放具有主导作用,在高P水平下沉积物中Fe与P的关系更加密切[17].
目前有关沉积物的研究主要基于主动采样技术,采样和运输过程会破坏沉积物原有性质,改变沉积物氧化还原条件[18],且分辨率低(cm级别),分析测定步骤繁琐且速度较慢.新型原位被动采样技术ZrO-Chelex薄膜扩散梯度技术(ZrO-Chelex DGT),可以在不破坏沉积物情况下,能够原位同步获取沉积物剖面有效态P和Fe的信息,分辨率达到毫米级. Xu等[19]和Sun等[20]通过室内模拟实验证明ZrO-Chelex DGT在自然界正常水体下能够同时测量沉积物剖面有效态P和Fe浓度,且其对这两种离子的检测极限浓度较高.
本研究选取长江中下游地区典型浅水湖泊太湖、巢湖、鄱阳湖和洞庭湖这4个湖泊为研究对象,利用ZrO-Chelex DGT同步获取现场沉积物水界面有效态P和Fe浓度的剖面分布信息,并对两者的变化进行分析,同时进行室内模拟实验,进一步明确铁磷耦合关系,为内源磷释放机理提供直接证据.
1 材料和方法 1.1 研究区概况太湖、巢湖、鄱阳湖和洞庭湖为中国四大淡水湖泊,他们分别位于江苏省南部、安徽省江淮丘陵中部、江西省北部以及湖南省东北部,其面积分别为2328、760、2933和2625 km2,平均深度分别为1.9、2.7、5.1和6.4 m.湖泊土壤类型基本为Fe、Mn元素富集的红壤或黄壤,湖水矿化度较高,主要为以Ca2+为主的阳离子以及以HCO3-为主的阴离子,其pH偏弱碱性,这些性质为蓝藻生长提供了适宜的环境[21].研究表明,这些湖泊均存在富营养化现象,且主要受农业非点源污染以及工业废水排放点源污染所致[22].
1.2 ZrO-Chelex DGT技术原理和装置准备薄膜梯度扩散技术(DGT)作为一种原位被动采样技术,由Davison等于1994年发明[23].该技术是以费克第一扩散定律为理论基础,当装置投放至水体或沉积物中,环境中自由态离子通过滤膜和扩散膜组成的扩散层,进而被固定膜捕获并累积,根据通量与费克第一定律公式计算出DGT有效浓度[24].
ZrO-Chelex DGT是在氧化锆DGT(Zr-oxide DGT)基础上发展起来的复合DGT,利用ZrO-Chelex凝胶层作为同步固定Fe、P的固定层[19, 25],其容量较高[19],还可同时测定P和As5+、Cr6+、Mo6+、Sb5+、Se6+、V5+、W6+等重金属离子[26-27]. ZrO-Chelex DGT购置于南京智感环境科技有限公司,采用了新型平板DGT塑料外套[28],固定膜的配制方法参考文献[19],扩散膜为1.5 %琼脂糖,厚度为0.8 mm,滤膜为PVDF膜(0.45 μm, 厚度为0.1 mm).组装DGT装置时,首先将固定膜放置在底板上,之后依次放置扩散膜和滤膜,最后盖板固定3层膜,置于去离子水充氮去氧16 h备用.
1.3 样品采集与分析方法 1.3.1 样品采集2015年5—7月,依次在太湖、巢湖、鄱阳湖和洞庭湖进行DGT装置投放和现场表层泥取样,其采样点分布见图 1.通过重力投放器,将DGT装置投放到采样点湖区沉积物中,24 h后回收DGT装置,标记沉积物水界面,用去离子水冲洗装置表面沉积物,装入自封袋保持湿润,带回实验室进行分析.采集太湖梅梁湾柱状样品和水样,低温保存带回实验室.其中柱状样进行现场分层,用于室内培养实验.

|
图 1 采样点分布 Fig.1 Distribution of sampling sites |
将太湖梅梁湾分层泥进行相同层次的混匀过筛,按沉积物分层顺序分装成4个平行沉积物柱状样,并添加上覆水(原位过滤水样),恒温25℃下淹水培养.室内模拟好氧厌氧环境,首先将稳定后的沉积物间断曝气使其充分好氧,随后加盖子密封,使沉积物逐渐厌氧,每天定时监测上覆水溶解氧浓度,在好氧3 d,厌氧7、14、30 d时投放DGT装置,同步获取沉积物剖面P、Fe的信息.
1.3.3 样品分析方法ZrO-Chelex DGT固定膜中有效态P和Fe浓度的测定参考文献[19]:沿DGT装置暴露窗口边缘划开,取出固定膜,用去离子水轻轻冲洗后吸去残留水,然后置于陶瓷组刀上将膜切成1 mm宽的长条,于400 μl 1 mol/L的HNO3提取液中,16 h后吸出提取液,待测有效态Fe;固定膜长条加入400 μl去离子水取出残留HNO3,加入400 μl 1 mol/L NaOH提取液,16 h后吸出提取液,待测有效态P浓度.提取液中PO43-和Fe2+浓度的测定分别采用钼蓝和邻菲罗啉微量比色法[29].
沉积物分析方法:将不同湖区的沉积物带回实验室后,称取约2 g沉积物样品烘干测定含水率.剩余样品封袋后马上进行低温冷冻,再将冻土进行冷冻干燥,研磨过筛后装入自封袋中待分析.沉积物各指标均按照标准分析方法分析[30].烧失量(LOI)通过在550℃环境下灼烧沉积物6 h测定;元素含量测定采用LiBO2消解法,提取液中总磷含量采用钼蓝显色法测定,其他金属离子含量用ICP-AES进行测定.
1.4 数据处理和分析根据PO43-和Fe2+微量比色的标准曲线,得到提取液中P和Fe的浓度,两者的累积量M为:
| $ M = \frac{{{C_{\rm{e}}}\left( {{V_{\rm{e}}} + {V_{\rm{g}}}} \right)}}{{{f_{\rm{e}}}}} $ | (1) |
式中,Ce为提取液中P和Fe的浓度,Ve和Vg分别为提取液和固定膜的体积,fe为提取率,ZrO-Chelex固定膜P和Fe的提取率分别为96 %和88 %.
有效态P和Fe浓度可依据公式(2)得到:
| $ {C_{{\rm{DGT}}}}{\rm{ = }}\frac{{M \cdot \Delta g}}{{{D_{\rm{g}}} \cdot A \cdot t}} $ | (2) |
式中,CDGT为沉积物剖面有效态浓度的平均值[31];Δg为扩散层厚度(cm);Dg为PO43-和Fe2+在扩散层中的扩散系数,分别为6.86和6.40(×10-6 cm2/s, 25℃),其值可参考文献[32];A为条状固定膜的面积(cm2);t为扩散时间(s).
2 结果与讨论 2.1 沉积物理化性质4个湖泊采样点表层沉积物的元素含量组成如表 1所示,Al和Fe含量最高,分别为64.9~75.7 mg/g和31.8~48.4 mg/g,其数值超过其他元素一个数量级. Ca含量仅次于Al和Fe,为2.1~6.4 mg/g. Mn在沉积物表层中的含量最低,为0.82~1.3 mg/g.沉积物总磷含量为0.54~0.84 mg/g,在各个湖泊的次序为洞庭湖>太湖>巢湖>鄱阳湖. LOI表征沉积物中有机质含量[33],太湖、巢湖、鄱阳湖和洞庭湖的LOI分别为4.7 %、6.8 %、6.6 %和4.9 %.研究表明,Fe/P比值可表征沉积物对内源磷负荷的控制能力[34],当Fe/P比值为10~15时,由于铁(氢)氧化物对磷的控制作用,内源P不足以释放.本研究4个湖泊Fe/P比值分别为71、52、59和46,表明所选采样点的沉积物内源磷的风险较小.
| 表 1 4个湖泊表层沉积物的基本理化性质 Tab.1 Basic physicochemical properties of sampling sites of the four lakes |
4个湖泊沉积物剖面DGT有效态P和Fe浓度分布如图 2所示.太湖、巢湖、鄱阳湖和洞庭湖的有效态P浓度均在沉积物水界面处增加,分别在-14、-10、-19和-26 mm处达到第1个峰值,且不同湖区沉积物峰值处有效态P的浓度不一,其中在鄱阳湖和洞庭湖浓度较高,分别为0.41和0.52 mg/L,与鄱阳湖和洞庭湖湖泊富营养化情况更为严峻一致[22].有效态P到达第一个峰值后继续波动变化,在不同深度达到峰谷值,且峰谷值数不一.峰值区域表明间隙水中有效态P浓度的增加[35].相应地,谷值区域可能为有效态P的释放衰减,且可能与细菌等微生物对有机质的降解有关,这种减少在时间上会持续到细菌衰亡[36-37].另外太湖和巢湖沉积物剖面有效态P浓度的垂向变化更为明显,这表明这两个湖泊沉积物异质性较强[38].有效态P浓度分别在太湖、巢湖和洞庭湖沉积物剖面的-53、-79和-56 mm处趋于平缓,且浓度值较高,可能与下层的还原环境有关系[39].

|
图 2 沉积物剖面有效态P和Fe浓度分布 Fig.2 Vertical distributions of labile P and Fe in the sediments profile |
4个湖泊沉积物剖面有效态Fe浓度范围变化较大,其增减幅度在峰谷处明显于有效态P浓度的增减幅度,这与Ding等[40]对太湖沉积物的原位测定结果一致.与有效态P浓度在沉积物剖面分布类似,有效态Fe浓度在沉积物剖面的垂向分布规律与有效态P一致,两者均在沉积物水界面处增加,并在相同深处波动变化,呈现同步升高或降低的趋势.
对上述4个剖面有效态P和Fe浓度变化明显区域(虚线界定区域)进行相关性分析,结果见图 3. 4个湖泊沉积物剖面有效态P和Fe浓度具有显著的正相关关系(P<0.05),表明沉积物中磷与铁均存在明显的耦合关系,磷释放受铁还原的控制,即好氧条件下Fe2+被氧化为Fe3+,所生成的(氢)氧化铁吸附固定磷,Fe和P同步减少;厌氧条件下Fe3+被还原为Fe2+,溶解释放Fe2+和P,有效态Fe和P同步增加[41].线性方程的斜率表征沉积物中有效态Fe对P迁移的能力[40].在太湖和鄱阳湖沉积物中,有效态Fe和P浓度比值的斜率较低(3.96和2.21),表明这两个湖泊沉积物中活性氧化铁浓度较低,沉积物剖面有效态P释放能力较强[40].而在巢湖和洞庭湖中,其斜率值较高(14.25和9.18),说明沉积物中对P的固定能力较强.

|
图 3 沉积物有效态P和Fe的相关性(*表示相关性显著,下同) Fig.3 Correlation analyses between labile P and Fe in sediments |
通过室内模拟好氧-厌氧环境,发现在沉积物厌氧过程中,有效态P和Fe浓度均在-10 mm左右处开始增加,于-40~-50 mm处达到峰值,且保持峰值的较高浓度,向下停止减少;随着厌氧过程的加剧,有效态P和Fe浓度逐渐同步升高(图 4).可见,随着沉积物内部还原环境的加强,铁不断还原,Fe3+被持续还原为Fe2+,铁结合态的磷也随之释放,导致了磷的释放量也逐渐增加.进一步分析不同厌氧条件下沉积物剖面有效态P和Fe浓度变化明显区域(虚线界定区域)的相关性,发现均呈显著或极显著的正相关,即两者在沉积物剖面存在同步释放的规律(图 5).室内厌氧培养实验进一步证实了磷铁之间的耦合关系,即铁的还原导致磷的释放. Ding等[40]对太湖14个位点进行原位测定,均发现沉积物剖面有效态P和Fe的释放具有同步性,与本研究的结果一致,很可能说明磷铁同步释放普遍存在于长江中下游湖泊. Rydin等[42]对波罗的海海岸沉积物界进行P释放机理探索,发现潜在活性P的释放主要受Fe-P控制,即P的释放受Fe的氧化还原控制[41, 43].可能的原因是Fe3+浓度的增加,会降低P的迁移性[44].另外,Fe2+浓度的减少,不仅仅受氧化还原环境的变化影响,也可能是硝酸或硫酸酶含量减少所致[45],硝酸盐细菌呼吸作用的增强,能够增加沉积物中Fe-P络合物中P释放的速率[46].同时,硝酸盐细菌的分泌物也能够加速Fe3+的溶解,使吸附在Fe(OH)3中的P释放出来[47].
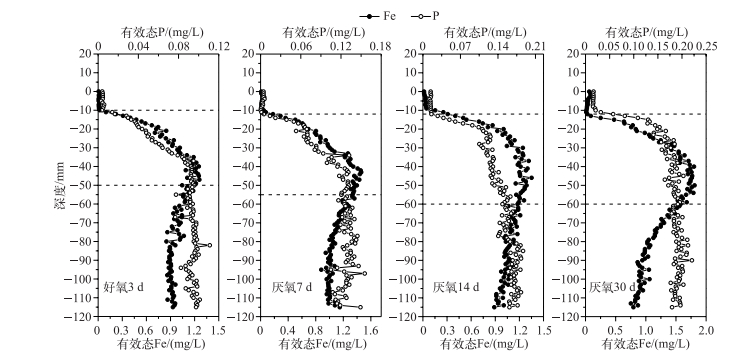
|
图 4 好氧-厌氧过程沉积物剖面有效态P和Fe的浓度分布 Fig.4 Distributions of labile P and Fe in the sediments during anaerobic-aerobic incubation |
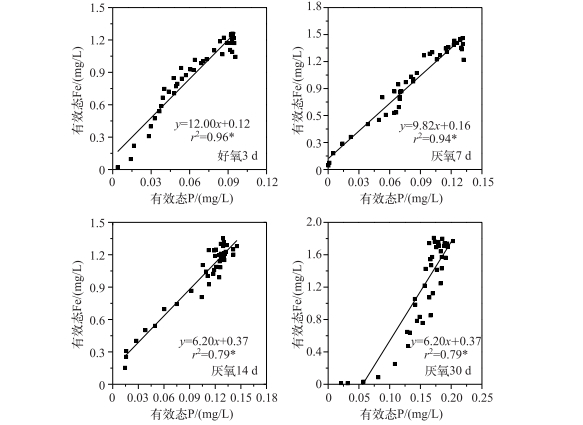
|
图 5 好氧-厌氧过程沉积物有效态P和Fe的相关性 Fig.5 Correlation analyses between labile P and Fe during anaerobic-aerobic incubation |
1) 不同湖泊沉积物理化性质差异较大,且磷铁含量在沉积物垂向分布具有较大的空间异质性,不同湖泊异质性也存在差异.
2) 沉积物中磷和铁存在耦合关系,磷、铁含量呈现同步增加或减少的趋势,4个湖泊沉积物均存在相同的变化规律.
3) 室内好氧厌氧培养实验验证了铁磷耦合关系,即铁的还原可导致磷释放,沉积物磷的二次迁移主要受铁氧化还原过程的控制.
| [1] |
Sondergaard M, Jensen PJ, Jeppesen E. Retention and internal loading of phosphorus in shallow, eutrophic lakes. Scientific World Journal, 2001, 1(1/2/3): 427-442. DOI:10.1100/tsw.2001.72 |
| [2] |
Xiao Yonghui. Continues online monitoring of eutrophication and early warning of cyanobacteria bloom in Chaohu lake[Dissertation]. Yangzhou:Yangzhou University, 2011. [肖永辉. 巢湖富营养化连续在线监测与蓝藻水华预警[学位论文]. 扬州: 扬州大学, 2011. http://cdmd.cnki.com.cn/Article/CDMD-11117-1013128671.htm ]
|
| [3] |
Wang Yan. Spatial and temporal distribution of nitrogen and phosphorus and ecological risk assessment of Dongting Lake[Dissertation]. Harbin:Northeast Forestry University, 2014. [王岩. 洞庭湖氮磷时空分布及生态风险评价[学位论文]. 哈尔滨: 东北林业大学, 2014. http://d.wanfangdata.com.cn/Thesis/Y2720955 ]
|
| [4] |
Cheng Fang. The study of eutrophication condition and aquatic community structure of Taihu Lake[Dissertation]. Suzhou:Soochow University, 2010. [成芳. 太湖水体富营养化与水生生物群落结构的研究[学位论文]. 苏州: 苏州大学, 2010. http://cdmd.cnki.com.cn/article/cdmd-10285-2010168354.htm ]
|
| [5] |
Sundareshwar PV, Morris JT, Koepfler EK et al. Phosphorus limitation of coastal ecosystem processes. Science, 2003, 299(5606): 563-565. DOI:10.1126/science.1079100 |
| [6] |
Cullen P, Forsberg C. Experiences with reducing point sources of phosphorus to lakes. Hydrobiologia, 1988, 170(1): 321-336. DOI:10.1007/BF00024912 |
| [7] |
Redshaw CJ, Mason CF, Hayes CR et al. Factors influencing phosphate exchange across the sediment-water interface of eutrophic reservoir. Hydrobiologia, 1990, 192(2): 233-245. DOI:10.1007/BF00006018 |
| [8] |
Xu D, Chen Y, Ding S et al. Diffusive gradients in thin films technique equipped with a mixed binding gel for simultaneous measurements of dissolved reactive phosphorus and dissolved iron. Environmental Science & Technology, 2013, 47(18): 10477-10484. DOI:10.1021/es401822x |
| [9] |
Meng Fande. Study on the relationship between physical and chemical properties and phosphorus along with their forms in the sediment of the mid-lower Yangtze Lakes[Dissertation]. Beijing:Capital Normal University, 2005. [孟凡德. 长江中下游湖泊沉积物理化性质与磷及其形态的关系研究[学位论文]. 北京: 首都师范大学, 2005. http://cdmd.cnki.com.cn/Article/CDMD-10028-2005073719.htm ]
|
| [10] |
Stockdale A, Davison W, Zhang H. High-resolution two-dimensional quantitative analysis of phosphorus, vanadium and arsenic, and qualitative analysis of sulfide, in a freshwater sediment. Environmental Chemistry, 2008, 5(2): 143-149. DOI:10.1071/EN07096 |
| [11] |
Jing L, Liu X, Bai S et al. Effects of sediment dredging on internal phosphorus:A comparative field study focused on iron and phosphorus forms in sediments. Ecological Engineering, 2015, 82: 267-271. DOI:10.1016/j.ecoleng.2015.04.099 |
| [12] |
Cesbron F, Metzger E, Launeau P et al. Simultaneous 2D imaging of dissolved iron and reactive phosphorus in sediment porewaters by thin-film and hyperspectral methods. Environmental Science & Technology, 2014, 48(5): 2816-2826. DOI:10.1021/es404724r |
| [13] |
Bortleson GC, Lee GF. Phosphorus, iron, and manganese distribution in sediment cores of six Wisconsin lakes 1. Limnology & Oceanography, 1974, 19(5): 794-801. |
| [14] |
Ostrofsky ML. Phosphorus species in the surficial sediments of lakes of eastern north America. Canadian Journal of Fisheries & Aquatic Sciences, 2011, 44(5): 960-966. DOI:10.1139/f87-114 |
| [15] |
Søndergaard M, Windolf J, Jeppesen E. Phosphorus fractions and profiles in the sediment of shallow Danish lakes as related to phosphorus load, sediment composition and lake chemistry. Water Research, 1996, 30(4): 992-1002. DOI:10.1016/0043-1354(95)00251-0 |
| [16] |
Smolders AJP, Lamers LPM, Moonen M et al. Controlling phosphate release from phosphate-enriched sediments by adding various iron compounds. Biogeochemistry, 2001, 54(2): 219-228. DOI:10.1023/A:1010660401527 |
| [17] |
Amirbahman A, Pearce AR, Bouchard RJ et al. Relationship between hypolimnetic phosphorus and iron release from eleven lakes in Maine, USA. Biogeochemistry, 2003, 65(3): 369-386. DOI:10.1023/A:1026245914721 |
| [18] |
Lange GJD, Cranston RE, Hydes DH et al. Extraction of pore water from marine sediments:A review of possible artifacts with pertinent examples from the North Atlantic. Marine Geology, 1992, 109(1/2): 53-76. DOI:10.1016/0025-3227(92)90220-C |
| [19] |
Xu D, Chen Y, Ding S et al. Diffusive gradients in thin films technique equipped with a mixed binding gel for simultaneous measurements of dissolved reactive phosphorus and dissolved iron. Environmental Science & Technology, 2013, 47(18): 10477-10484. DOI:10.1021/es401822x |
| [20] |
Sun Q, Zhang L, Ding S et al. Evaluation of the diffusive gradients in thin films technique using a mixed binding gel for measuring iron, phosphorus and arsenic in the environment. Environmental Science Processes & Impacts, 2015, 17(3): 1143-1147. DOI:10.1039/c4em00629a |
| [21] |
Wang Qi. Study on the biological mechanism of phosphorus release from sediments in the shallow lakes[Dissertation]. Xi'an:Northwest A & F University, 2006. [王琦. 浅水湖泊沉积物磷释放的生物学机制研究[学位论文]. 西安: 西北农林科技大学, 2006. http://d.wanfangdata.com.cn/Thesis/Y977673 ]
|
| [22] |
Zhang Binliang. The characteristics of phosphorus behavior at sediment-water interface and environmental risk assessment from sallow lakes[Dissertation]. Shanghai:East China Normal University, 2004. [张斌亮. 浅水湖泊沉积物水界面磷的行为特征与环境风险评价[学位论文]. 上海: 华东师范大学, 2004. http://cdmd.cnki.com.cn/article/cdmd-10269-2004087674.htm ]
|
| [23] |
Davison W, Zhang H. Progress in understanding the use of diffusive gradients in thin films (DGT)-back to basics. Environmental Chemistry, 2012, 9(1): 1-13. DOI:10.1071/EN11084 |
| [24] |
Zhang C, Ding S, Xu D et al. Bioavailability assessment of phosphorus and metals in soils and sediments:a review of diffusive gradients in thin films (DGT). Environmental Monitoring & Assessment, 2014, 186(11): 7367-7378. DOI:10.1007/s10661-014-3933-0 |
| [25] |
Ding S, Xu D, Sun Q et al. Measurement of dissolved reactive phosphorus using the diffusive gradients in thin films technique with a high-capacity binding phase. Environmental Science & Technology, 2010, 44(21): 8169-8174. DOI:10.1021/es1020873 |
| [26] |
Ding S, Xu D, Wang Y et al. Simultaneous measurements of eight oxyanions using high-capacity diffusive gradients in thin films (Zr-oxide DGT) with a high-efficiency elution procedure. Environmental Science & Technology, 2016, 50(14): 7572-7580. DOI:10.1021/acs.est.6b00206 |
| [27] |
Chen M, Ding S, Zhang L et al. An investigation of the effects of elevated phosphorus in water on the release of heavy metals in sediments at a high resolution. Science of the Total Environment, 2017, 575: 330-337. DOI:10.1016/j.scitotenv.2016.10.063 |
| [28] |
Ding SM, Wang Y, Zhang LP et al. New holder configurations for use in diffusive gradients in thin films (DGT) technique. RSC Advance, 2016, 6(91): 88143-88156. DOI:10.1039/c6ra19677b |
| [29] |
Wang Yan, Zhu Chungang, Xu Di et al. Development of a method for measurement dissolved reactive phosphorus (DRP) and dissolved ferrous iron in large batch in pore water samples of sediments with micro-volumes. Environmental Science, 2014, 35(4): 1271-1277. [王燕, 朱春刚, 许笛等. 一种大批量测定沉积物微量间隙水样品中溶解态磷和铁含量的方法. 环境科学, 2014, 35(4): 1271-1277.] |
| [30] |
Lu Rukun. Soil agricultural chemical analysis method. Beijing: China Agriculture Scientech Press, 2000. [鲁如坤. 土壤农业化学分析方法. 北京: 中国农业科技出版社, 2000.]
|
| [31] |
Luo Jun, Wang Xiaorong, Zhang Hao et al. Theory and application of diffusive gradients in thin films in soils. Journal of Agro-Environment Science, 2011, 30(2): 205-213. [罗军, 王晓蓉, 张昊等. 梯度扩散薄膜技术(DGT)的理论及其在环境中的应用Ⅰ:工作原理、特性与在土壤中的应用. 农业环境科学学报, 2011, 30(2): 205-213.] |
| [32] |
Li YH, Gregory S. Diffusion of ions in sea water and in deep-sea sediments. Geochimica et Cosmochimica Acta, 1974, 38(5): 703-714. DOI:10.1016/0016-7037(74)90145-8 |
| [33] |
Liu Ziting, Yu Junqing, Zhang Baohua et al. Application of loss on ignition to the study of lake sediments and environmental changes. Journal of Salt Lake Research, 2006, 14(2): 67-72. [刘子亭, 余俊清, 张保华等. 烧失量分析在湖泊沉积与环境变化研究中的应用. 盐湖研究, 2006, 14(2): 67-72.] |
| [34] |
Caraco NF, Cole JJ, Likens GE. Sulfate control of phosphorus availability in lakes. Hydrobiologia, 1993, 253(1): 275-280. DOI:10.1016/j.scitotenv.2015.10.155 |
| [35] |
Wang Yanping, Guan Qingwei, Li Chao et al. A study of in situ synchronous measurement of available phosphorus and sulfur in the sediments of lake Chaohu by diffusive gradients in thin films(DGT). Acta Scientiae Circumstantiae,, 2015, 35(8): 2512-2518. [王艳平, 关庆伟, 李超等. 巢湖沉积物有效态磷与硫的DGT原位同步分析研究. 环境科学学报, 2015, 35(8): 2512-2518. DOI:10.13671/j.hjkxxb.2014.0996] |
| [36] |
Tezuka Y. Bacterial regeneration of ammonium and phosphate as affected by the carbon:nitrogen:phosphorus ratio of organic substrates. Microbial Ecology, 1990, 19(3): 227-238. DOI:10.1007/BF02017167 |
| [37] |
Hessen DO, Ågren GI, Anderson TR et al. Carbon sequestration in ecosystems:the role of stoichiometry. Ecology, 2004, 85(5): 1179-1192. DOI:10.1890/02-0251 |
| [38] |
Ding S, Chao H, Wang Y et al. In situ, high-resolution imaging of labile phosphorus in sediments of a large eutrophic lake. Water Research, 2015, 74: 100-109. DOI:10.1016/j.watres.2015.02.008 |
| [39] |
Ding S, Wang Y, Xu D et al. Gel-based coloration technique for the submillimeter-scale imaging of labile phosphorus in sediments and soils with diffusive gradients in thin films. Environmental Science & Technology, 2013, 47(14): 7821-7829. DOI:10.1021/es400192j |
| [40] |
Ding S, Wang Y, Wang D et al. In situ, high-resolution evidence for iron-coupled mobilization of phosphorus in sediments. Scientific Reports, 2016. DOI:10.1038/srep24341 |
| [41] |
Baccini P. Phosphate interactions at the sediment-water interface. In:Stumm W ed. Chemical processes in lakes. New York: Wiley-Interscience, 1985, 189-205.
|
| [42] |
Rydin E, Malmaeus JM, Karlsson OM et al. Phosphorus release from coastal Baltic Sea sediments as estimated from sediment profiles. Estuarine Coastal & Shelf Science, 2011, 92(1): 111-117. DOI:10.1016/j.ecss.2010.12.020 |
| [43] |
Mortimer CH. The exchange of dissolved substances between mud and water in lakes. Ⅰ & Ⅱ J Ecol, 1941, 30(21): 147-201. DOI:10.2307/2256691 |
| [44] |
Jones JGS, Gardener S, Simon BM. Bacterial reduction of ferric iron in a stratified eutrophic lake. Microbiology, 1983, 129(1): 131-139. DOI:10.1099/00221287-129-1-131 |
| [45] |
Sperber JI. Release of phosphate from soil minerals by hydrogen sulphide. Nature, 1958, 181(4613): 934. DOI:10.1038/181934a0 |
| [46] |
Jansson M. Nitrate as a catalyst for phosphorus mobilization in lake sediments. In:Sly PA ed. Sediments and water interactions. New York: Springer-Verlag, 1986, 382-387.
|
| [47] |
Jansson M. Anaerobic dissolution of iron-phosphorus complexes in sediment due to the activity of nitrate-reducing bacteria. Microbial Ecology, 1987, 14(1): 81-89. DOI:10.1007/BF02011573 |
 2017, Vol. 29
2017, Vol. 29 

