(2: 中国科学院南京地理与湖泊研究所湖泊与环境国家重点实验室, 南京 210008)
(3: 云南大学国际河流与生态安全研究院, 昆明 650031)
(2: State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing 210008, P. R. China)
(3: Institute of International Rivers and Eco-Security, Yunnan University, Kunming 650031, P. R. China)
氮、磷等营养盐的过度输入引起的水体富营养化是世界上最广泛关注的水环境问题之一.营养盐的输入导致浮游植物的异常增殖,出现蓝藻水华.藻华消退过程会产生大量的溶解性有机质(称为藻源溶解性有机质,algae derived-dissolved organic matter, A-DOM)[1],A-DOM很快就会消耗水体中的溶解氧(DO),使得厌氧条件下藻类代谢产生二甲基异莰醇等恶臭物质,严重危及到人们饮用水安全[2].因此,加快A-DOM的降解,有利于消除藻华暴发对饮用水安全的不利影响.光化学降解是DOM重要的降解途径;相对于生物降解,光化学降解的速度更快[3].
由于DOM成分复杂,目前很难以对其进行全面的分析.近年来发展起来的三维荧光光谱-平行因子分析法(EEMs-PARAFAC)能够很好区分DOM的组分和来源[4].利用EEMs-PARAFAC技术,可以研究各EEMs-PARAFAC组分的光反应性和其在反应过程中的变化[5-10].但是,在不同研究中,一些相似组分却表现出不同的光反应性.例如Stedmon等[11]研究了浮游植物群落产生的DOM的光降解,发现类色氨酸(类蛋白质的一种)能被光降解;但张运林等[12]认为类蛋白质难以被光降解.以上研究的不一致可能在于实验条件的差异,Stedmon采用了人工光源,而张运林等利用自然光照射;且两个研究中DOM的EEMs-PARAFAC组分有细微差异.
可见,反应条件对DOM的光化学降解有着重要的影响;有机物的光化学降解过程可能受各种条件(包括光源、光强、pH、载气等)的影响[13-14].但目前,关于反应条件对A-DOM的EEMs-PARAFAC组分光化学降解的影响方面的研究较少.明确A-DOM的降解效率的影响因素能为消除藻华暴发对饮用水的影响提供理论依据;也有助于了解A-DOM的迁移转化过程.因此,本文以A-DOM为研究对象,利用EEMs-PARAFAC,研究A-DOM的光化学降解;并通过控制不同实验条件(光照强度、照射光波长、DOC浓度和载气),来研究各参数对A-DOM降解的影响.结果揭示了A-DOM各组分的光反应性及不同条件对其光化学降解的影响,有助于了解A-DOM的迁移转化,能为湖泊蓝藻水华暴发时应急处理和保障饮用水安全提供依据.
1 材料与方法 1.1 A-DOM的提取于2016年6月14日在中国科学院太湖湖泊生态系统试验站(31°25.42′N,120°12.57′E)采集含蓝藻水样10 L,再通过浮游植物网过滤浓缩得到含蓝藻浓度较高的水样;对浓缩后的藻水进行离心(9000转/min,15 min),分离出藻浆;然后将藻浆放入超纯水中混合清洗,再离心出藻浆,重复3遍;最后用液氮将蓝藻快速冷冻,再解冻,重复3遍,将蓝藻细胞打破[15].处理后的溶液经孔径为0.22 μm的滤膜(Millipore,PES Membrane)过滤得到A-DOM溶液,此溶液为A-DOM的储备液,冷藏.经测定,储备液浓度为550 mg C/L.
1.2 A-DOM的光降解光化学降解反应在室温条件下的多试管搅拌型光反应仪(南京胥江,图 1a)中进行.激发光源采用500 W和300 W的中压汞灯,其发射光谱见图 1b;根据发射光谱图计算,光强分别为391.7和91.5 W/m2.在紫外和可见光波段光强分别为140.3、251.4 W/m2(500 W汞灯),30.2、61.3 W/m2(300 W汞灯);反应前将一定体积的A-DOM储备液配制成500 ml 10 mg C/L的溶液;使用0.1 mol/L的HCl和0.1 mol/L的NaOH调节pH;将每50 ml配制好的溶液加入9个石英反应管中,开启冷却水装置,控制温度为28℃左右;待系统稳定后,打开汞灯光源,以此作为反应起点.在特定反应时间(0、0.5、1、2、4、6、8、10、12 h)各取出一个石英反应管.取出的水样放入冰箱中冷藏,在两周内进行紫外-可见光全波扫描(UV-Vis)、三维荧光光谱(EEMs)分析.
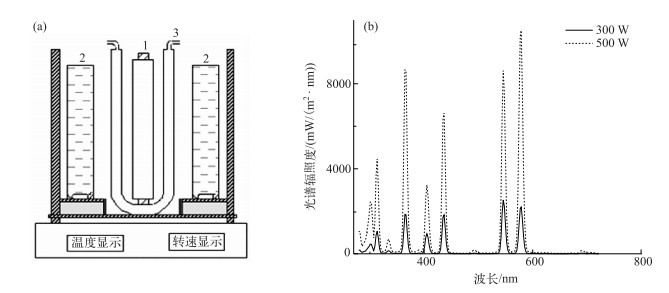
|
图 1 光降解反应器示意图(a)(1:光源;2:石英反应管;3:石英冷阱)及中压汞灯发射光谱图(b) Fig.1 The schematic of photodegradation reaction instrument(a)(1-lamp; 2-water-cooled jacket; 3-quartz tube)and the emission spectrum of median-pressure spectrum of mercury lamp(b) |
通入载气(O2或者N2)实验,在开启冷却水后,往反应管中通入O2或者N2 30 min,使得溶液中DO接近饱和或约为0;再开启汞灯启动实验;实验过程一直通入载气.
1.3 A-DOM的分析紫外-可见光全波扫描光谱(UV-Vis Spectra):使用Lambda 35(Perkin Elmer)紫外可见分光光度计,测试范围为200~800 nm,参比为Milli-Q水.根据式(1)和(2),通过一定波长下的吸光度计算得到该波长下的吸收系数[16]:
| $ a(\lambda \prime ){\rm{ }} = 2.303D(\lambda )/r $ | (1) |
式中, a(λ′)为波长λ下未校正的吸收系数(m-1);D (λ)为吸光度;r为光程路径(0.01 m).由于过滤清液可能残留细小颗粒会引起散射, 为此作如下散射效应订正[17]:
| $ a(\lambda ){\rm{ }} = a(\lambda \prime ){\rm{ }}-a\left( {750} \right)\lambda /750 $ | (2) |
式中, a (λ)为波长λ的吸收系数(m-1);λ为波长(nm).
采用光谱斜率比值(SR)表征A-DOM分子量的变化,SR与相对分子量呈反比[18-19],其计算方法为:
| $ {S_{\rm{R}}} = {S_{(275-295)}}/{S_{(350-400)}} $ | (3) |
式中, S(275-295)和S(350-400)分别为波长275~295 nm和350~400 nm的光谱斜率(nm-1).
EEMs采用日立F-7000荧光光谱仪测定(Hitachi High-Technologies).激发波长为200~450 nm,以5 nm为间隔;发射波长为250~550 nm,以1 nm为间隔.仪器校正根据日立F-7000说明书进行[20]. EEMs-PARAFAC模型的建立:首先对三维光谱进行校正:(1)根据McKnight等利用紫外可见吸收光谱数据进行内滤效应修正[21];(2)用Milli-Q水做空白扣除;(3)利用Milli-Q水的拉曼单位归一化(R.U.)来校准日常的荧光强度的变化.再参考Stedmon等[22]提出的方法,利用DOMFluor工具箱在MATLAB R2008a软件中得到4个组分模型(C1~C4),通过折半分析(split-half analysis)和随机初始化(random initialization)方法来验证模拟结果的可靠性,识别出4个组分的荧光强度.
此外利用拟一级降解动力学方程(式(4))来确定各个PARAFAC组分的降解速率[23]:
| $ {\rm{ln}}\;C/{C_0} = - {\rm{k}} \cdot t + {\rm{A}} $ | (4) |
式中,C为某一光照时间PARAFAC组分的荧光强度(R.U.);C0为该组分的初始荧光强度(R.U.);t为光照时间(h);k为拟一级降解动力学常数(h-1),k值越大表示光化学降解率越快;A为常数.
2 结果与讨论 2.1 A-DOM的EEMs-PARAFAC组分根据不同光照条件实验获得的EEMs数据,建立了4个组分(C1~C4)模型.其三维荧光光谱图及激发、发射波长特征图见图 2. 表 1列出了C1~C4激发和发射波长峰值及分类.

|
图 2 平行因子分析得到的4个组分三维荧光光谱图(a~d)及激发、发射波长特征图(e~h) Fig.2 EEMs contours (a-d) and the spectral characteristics of the EEMs-PARAFAC components (e-h) |
| 表 1 C1~C4激发和发射波长峰值及分类 Tab.1 Excitation and emission maxima of the four PARAFAC components and possible assignments |
10 mg C/L的A-DOM中C1~C4的荧光强度分别为2.13、0.82、6.52和0.11 R.U.,各组分在A-DOM荧光贡献百分比分别是22.2 %、8.6 %、68.1 %和1.1 %,说明A-DOM的荧光贡献中,以含类色氨酸基团的组分为主,还有部分UVA、UVC类腐殖质.
2.2 A-DOM的光降解行为研究从光照过程A-DOM的UV-Vis光谱变化可以看出, 未经光照(0 h)的A-DOM有两个吸收峰,分别在260和330 nm; A-DOM对紫外光吸收较为明显, 而对于可见光几乎不吸收.随着实验的进行,A-DOM的吸光度不断减小,最大吸收波长的吸光度在200~355 nm处下降最为明显(图 3).为了定量地描述光化学降解的过程,我们采用355 nm处的吸收系数(a355)的变化和降解率(降解率(%)=[1-[a355]/[a355]0]×100)来表征A-DOM的降解(图 3a).可见a355在前2 h内降解速度很快,反应初始a355为3.16,经过2个小时的光化学降解后吸光系数为1.86 m-1,降解率为41.1 %;2 h之后,a355的减少速率变缓;12 h后吸光系数为0.94 m-1,a355降解率为70.4 %. SR在光化学降解的前4 h内由开始的1.45迅速上升至2.91, 之后逐渐趋于平缓,说明光化学降解的过程中A-DOM的分子量会逐渐减小,大分子DOM裂解为小分子DOM(图 3b).
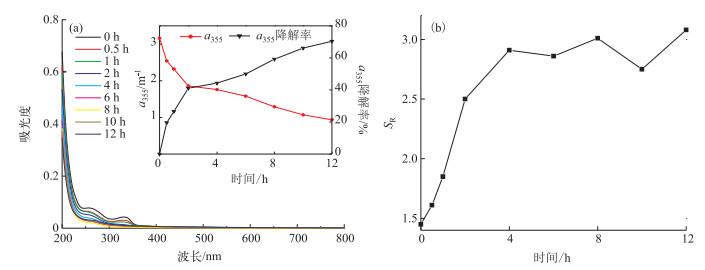
|
图 3 A-DOM降解过程UV-Vis光谱(a)(光源=500 W汞灯;不加滤光膜;pH=8.0;T=28℃;无载气;[DOC]0=10 mg/L)及SR的变化(b) Fig.3 Changes of UV-Vis spectra during the photodegradation of A-DOM(a)(500 W mercury lamp; without filtered membrane; pH=8.0; T=28℃; no carrier gas; [DOC]0=10 mg/L)and spectral slope ratio(b) |
为了更好地了解光化学降解各组分的变化过程,我们将EEMs-PARAFAC模型的3个组分(C1~C3)作图分析(图 4).可以看出,C1~C3组分在光照过程中呈下降趋势.对比C1、C2和C3的降解,类蛋白质组分C3的降解速率最快,经0.5 h的光照,其降解率为89 %;在整个光照过程中,其降解率为99.9 %,说明C3组分光反应性很强,极易发生光化学降解;这和Stedmon的报道是一致的.对于两种类腐殖组分C1和C2,C1组分在反应的前4 h快速降解,t=4 h时降解率为83.4 %,4 h之后,降解速率趋于平缓;12 h时,C1的降解率为96.1 %.相对于C1,C2的降解速率较为缓慢,整个实验的光化学降解率为85 %,降解过程中呈下降趋势,未出现明显的先快速降解再缓慢降解的趋势. C1比C2拥有更长的激发波长;这与文献[29]中报道的一致:有更长激发波长的类腐殖质的光降解速度快于短激发波长的类腐殖质.通过拟合出各组分的光化学降解动力学常数,来分析各组分的的光反应性. C1、C2和C3降解动力学常数分别为0.2848、0.1568、0.4611(R2>0.9),降解动力学常数越大其降解速率也就越快,由此进一步证明了三者的光反应性为C3>C1>C2.由于C4(类络氨酸)初始的荧光强度为0.109 R.U.,在A-DOM的荧光贡献中占比仅为1.1 %,且在后续光化学降解过程中荧光强度变化不大;在12 h的光化学降解过程后,其荧光强度微微上升至0.118 R.U.,由此可以推断光照对C4组分浓度无较大影响,因此在光化学降解过程中不加以分析.
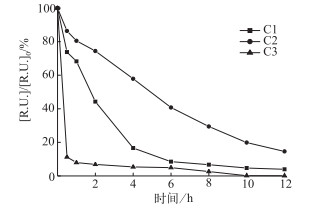
|
图 4 光化学降解过程C1、C2和C3组分的变化(光源=500 W汞灯;不加滤光膜;pH=8.0;T=28℃;无载气;[DOC]0=10 mg/L) Fig.4 Changes of C1, C2 and C3 during the photochemical degradation(500 W mercury lamp; without filtered membrane; pH=8.0; T=28℃; no carrier gas; [DOC]0=10 mg/L) |
图 5a显示了不同光强(500和300 W汞灯)A-DOM降解过程a355的变化情况,其纵坐标代表的是不同时间下a355相对于[a355]0(初始a355)的比值.可见,随着光强的升高,a355的降解速率加快,由此推断出A-DOM的降解速率加快.在500和300 W汞灯光照条件下,反应12 h后a355降解率分别为70.4 %和53.1 %.由此可见,光照强度对于光化学降解有明显的影响,光照强度越高,其总的光化学降解速率越快,降解率越高.这可能是随着光强的增大,提供的光子增多,A-DOM能吸收的光子增多,根据光降解原理[30-31],更多的A-DOM被激发,继而发生光裂解而被降解.

|
图 5 光照强度对A-DOM光降解的影响(不加滤光膜;pH=8.0;T=28℃;无载气;[DOC]0=10 mg/L) Fig.5 Effect of light intensity on the photodegradation of A-DOM (without filtered membrane; pH=8.0; T=28℃; no carrier gas; [DOC]0=10 mg/L) |
图 5b~d显示了光强对3个组分降解的影响, 其纵坐标代表的是不同时间下C1、C2和C3相对于各组分初始荧光强度的比值;每个组分在两种光强下的降解趋势基本一致,但降解速率有明显区别.随着光强的减弱,(从500 W到300 W),组分的降解率减少;在500和300 W汞灯光照下,C1的光化学降解率分别为96.1 %和77.4 %,降解动力学常数为0.2848和0.1301(R2>0.9);C2光化学降解率为85.4 %和72.8 %,降解动力学常数为0.1568和0.1055(R2>0.9);C3光化学降解率为99.2 %和98.5 %,降解动力学常数为0.4611和0.2431(R2>0.8).由降解动力学常数可知,在500和300 W汞灯光照下,3个组分的降解速率均为C3>C1>C2.可见,光强对组分的光反应性并没有影响.光源为500 W汞灯时,光强约为300 W汞灯的4倍,但3个组分的降解速率却只增加1倍.这说明随着光强增大,虽然降解速度提高,但更多的能量被浪费.在利用光强控制A-DOM的降解时,需要结合成本和实际情况加以考虑.
3.2 波长的影响为了考察波长对降解的影响,在冷肼的外面包裹不同类型的滤光膜(滤膜1、滤膜2),滤膜1滤去UVB波段(280~320 nm)的紫外光,滤膜2滤去UVB(280~320 nm)和UVA波长(320~400 nm)的紫外光.无滤膜、包裹滤膜1、包裹滤膜2的处理组分别称为Vis+UVA+UVB、Vis+UVA和Vis处理组.
经12 h光照后,Vis+UVA+UVB和Vis+UVA处理组中的a355降解率分别为70.4 %、31.9 %,而Vis处理组中a355在误差范围内几乎无变化. A-DOM在Vis+UVA+UVB下光降解速率最快且光化学降解率最高, 在Vis处理组几乎没有降解; 由此推断可以使A-DOM发生光化学降解的波长主要集中在紫外光区;UV光的波长短、能量高,是光化学降解主要的能量来源光区.对比Vis+UVA+UVB和Vis+UVA组,在滤去UVB波长光后,A-DOM先降解后趋于平缓不再降解,说明相对UVA光区,UVB光区对光化学降解的贡献较大(图 6a). Vis+UVA+UVB、Vis+UVA和Vis处理组A-DOM各组分也表现出了类似的规律,C1的降解率分别为96.1 %、41.1 %和2.1 %;C2的降解率分别为85.4 %、48.7 %、0.03 %;C3的降解率分别为99.9 %、74.8 %和32.7 % (图 6b~d).在滤去UVA或UVA+UVB的处理组,已不符合一级降解动力学方程,进一步证明紫外光对各组分的光化学降解有较大的影响.通过图 6可以看出,在Vis处理组中,C1和C2在去除UV光后几乎无降解,而在Vis+UVA+UVB和Vis+UVA处理组均有降解,由此可以推断出C1、C2的光化学降解光区为UV区.在滤去UV光后,32.7 %的C3组分被降解,由此推断可见光和紫外光均能降解C3组分,但紫外光的降解能力更强.
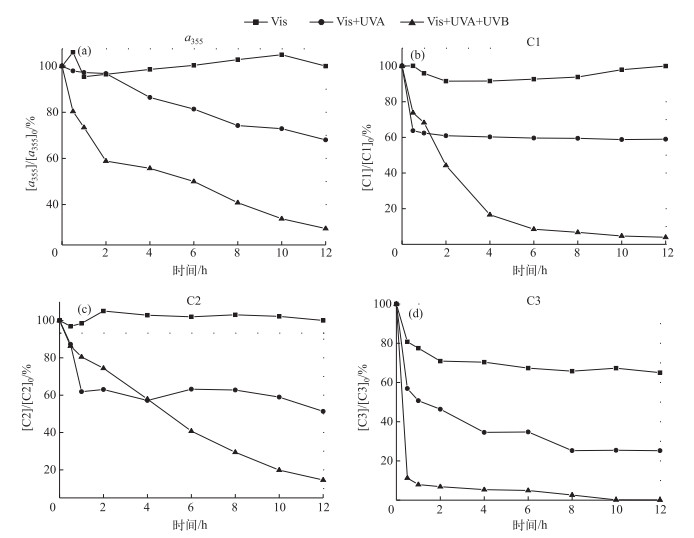
|
图 6 不同处理组波长对A-DOM光降解的影响 (光源=500 W汞灯;pH=8.0;T=28℃;无载气;[DOC]0=10 mg/L) Fig.6 Effect of the irradiation wavelength on the photodegradation of A-DOM (500 W mercury lamp; pH=8.0; T=28℃; no carrier gas; [DOC]0=10 mg/L) |
当初始DOC浓度分别为5、10、15和20 mg/L的A-DOM溶液,经过汞灯12 h的光化学降解,a355的降解率分别为86.2 %、70.4 %、68.3 %和65.1 % (图 7).相同条件下A-DOM浓度越低越容易降解,其总的光化学降解率越高.当初始DOC浓度分别为5、10、15和20 mg/L时,C1的降解率分别为99.4 %、99.2 %、96.8 %和91.1 %,降解动力学常数分别为0.4517、0.4305、0.3824和0.3119(R2>0.9);C2降解率分别为96.3 %、91.6 %、85.9 %和73.2 %,降解动力学常数分别为0.2998、0.1609、0.1568和0.1114(R2>0.9);C3降解率分别为99.6 %、99.3 %、98.7 %和98.3 %,降解动力学常数分别为0.4611、0.4343、0.4079和0.354(R2>0.8).可见,3种PARAFAC组分中,C2的光化学降解受浓度的影响最为显著,浓度越高降解速率越低且降解率也越低;C1降解受浓度影响并不是特别明显;C3组分的光化学降解受浓度的影响最小,由于3组分的光反应性为C3>C1>C2,C2相对于C3、C1光反应性最弱.当光强一定时,C3和C1对光的能量吸收处于优势,容易吸收光的能量发生光解,而被C2吸收光较少,因此C2受A-DOM的浓度影响最为显著.
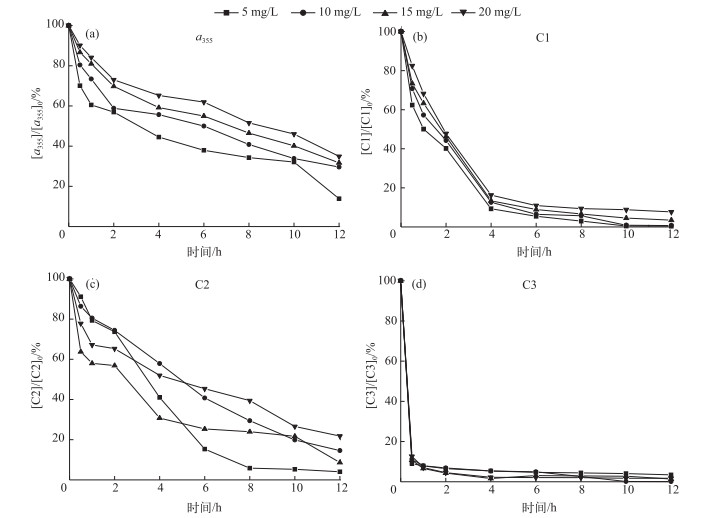
|
图 7 A-DOM浓度对其光降解的影响(光源=500 W汞灯;不加滤光膜;pH=8.0;T=28℃;无载气) Fig.7 Effect of A-DOM concentration on its photodegradation (500 W mercury lamp; without filtered membrane; pH=8.0; T=28℃; no carrier gas) |
通过向光照溶液中通入不同载气的方式来控制水样的DO浓度;通入氧气和氮气,DO浓度为饱和、接近于0的水平.不通载气时,DO浓度处在两者之间.通氧气、不通载气、通氮气条件下,经过12 h的光化学降解A-DOM的a355降解率分别为85.6 %、77.4 %、70.4 %.由图 8可以看出,DO浓度越高,A-DOM光化学降解的速率越快,且光照12 h后,总的光化学降解率也越高.通过EEMs-PARAFAC来分析DO对A-DOM各组分光化学降解速率的影响.在通氧气、不通载气和通氮气的条件下,12 h光照后,C1组分降解率分别为96.3 %、96.1 %和93.1 %,降解动力学常数分别为0.2824、0.2614和0.2094(R2>0.9);C2组分降解率分别为91.2 %、85.5 %和63.4 %,降解动力学常数分别为0.1586、0.1233和0.0769(R2>0.9);C3组分降解率分别为99.9 %、99.9 %和92.8 %,降解动力学常数分别为0.4615、0.4417、0.4303(R2>0.7).有机物的光化学降解有两个途径,一是直接光降解,即溶解性有机质直接吸收光的能量发生的降解变化;另一个是间接光解,通过光照过程中生成的活性氧物质(reactive oxygen species,ROS)的氧化作用;水中存在的中间介质吸收光子经过电子转移过程将能量传递给有机质,激发态的有机质和氧气反应生成ROS[32-33].可见,DO的存在对间接光解起到决定性作用,除去DO,有机质只能通过直接光解途径被降解.对C1、C2、C3组分降解进行对比发现,C3组分表现出较小的差异,说明直接光解(通N2)能很好地降解C3;而间接光解(通O2)对C3的降解影响很小. DO对难降解组分C1和C2有很好的促进作用,间接光解是C1和C2的重要降解途径.
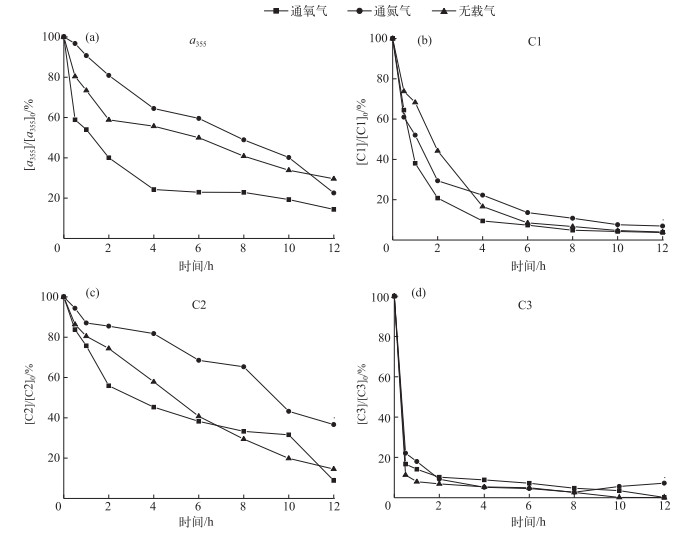
|
图 8 DO浓度对A-DOM光降解的影响 (光源=500 W汞灯;不加滤光膜;pH=8.0;T=28℃;[DOC]0=10 mg/L) Fig.8 Effect of dissolved oxygen concentration on the photodegradation of A-DOM (500 W mercury lamp; without filtered membrane; pH=8.0; T=28℃; [DOC]0=10 mg/L) |
1) 本文通过A-DOM光化学降解,建立EEMs-PARAFAC组分模型,鉴定A-DOM光化学降解过程中出现的4个荧光组分:C1(UVC类腐殖质)、C2(UVA类腐殖质)、C3(类色氨酸)、C4(类络氨酸);其对总荧光强度的贡献分别为8.6 %、22.2 %、68.1 %和1.1 %.
2) 当以500 W中压汞灯为光源时,pH=8.0,反应温度为28℃,光照12 h后a355降解率为70.4 %,随着光照时间的增加,A-DOM的吸光度不断下降且分子量逐渐减小.各EEMs-PARAFAC组分的光反应性为C3>C1>C2.
3) 紫外光、光强、DO的增加均能加速A-DOM的光化学降解.对于较难降解的C1和C2组分,可见光不能使其降解,其只能在紫外光下降解,而C3组分在可见光和紫外光区都能降解;光强增加对3个组分的降解均有促进作用,但降解速率的增加倍数小于光强增加的倍数;DO的引入能很好地促进C1和C2的降解.因此,在消除蓝藻暴发产生的大量A-DOM以防止对饮用水安全产生危害时,可结合实际情况,选择外加紫外光源、通入DO或者增加光源强度等手段来加速A-DOM的降解.
| [1] |
Li K, Xing B, Torello WA. Effect of organic fertilizers derived dissolved organic matter on pesticide sorption and leaching. Environmental Pollution, 2005, 134(2): 187-194. DOI:10.1016/j.envpol.2004.08.011 |
| [2] |
Yang M, Yu J, Li Z et al. Taihu Lake not to blame for Wuxi's Woes. Science, 2008, 319(5860): 158. DOI:10.1371/journal.phone.0051976 |
| [3] |
Stedmon CA, Markager S. Tracing the production and degradation of autochthonous fractions of dissolved organic matter by fluorescence analysis. Limnology and Oceanography, 2005, 50(5): 1415-1426. DOI:10.4319/lo.2005.50.5.1415 |
| [4] |
Stedmon CA, Markager S. Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnology and Oceanography, 2005, 50(2): 686-697. DOI:10.4319/lo.2005.50.2.0686 |
| [5] |
Gao J, Jiang T, Yan JL et al. Photobleaching of dissolved organic matter (DOM) from confluence of two rivers under natural solar radiation:A case study of Fujiang River-Jialingjiang River. Environmental Science, 2014, 35(9): 3397-3407. [高洁, 江韬, 闫金龙等. 天然日光辐照下两江交汇处溶解性有机质(DOM)光漂白过程:以涪江——嘉陵江为例. 环境科学, 2014, 35(9): 3397-3407. DOI:10.13227/j.hjkx.2014.09.021] |
| [6] |
Du Y, Zhang Y, Chen F et al. Photochemical reactivities of dissolved organic matter (DOM) in a sub-alpine lake revealed by EEM-PARAFAC:An insight into the fate of allochthonous DOM in alpine lakes affected by climate change. Science of the Total Environment, 2016, 568: 216-225. DOI:10.1016/j.scitotenv.2016.06.036 |
| [7] |
Xu H, Jiang H. UV-induced photochemical heterogeneity of dissolved and attached organic matter associated with cyanobacterial bloomsina eutrophic freshwater lake. Water Research, 2013, 47(17): 6506-6515. DOI:10.1016/j.watres.2013.08.021 |
| [8] |
Zhang Y, Liu M, Qin B et al. Photochemical degradation of chromophoric-dissolved organic matter exposed to simulated UV-B and natural solar radiation. Hydrobiologia, 2009, 627(1): 159-168. DOI:10.1007/s10750-009-9722-z |
| [9] |
Zhang Y, Hu CM, Yu T. Photodegradation of chromophoric dissolved organic matters in the water of Lake Dianchi, China. Frontiers of Environmental Science and Engineering, 2014, 9(4): 575-582. DOI:10.1007/s11783-014-0664-y |
| [10] |
Guo WD, Cheng YY. Photodegradation of chromophoric dissolved organic matter from Jiulong River Estuary under natural solar radiation. Environmental Science, 2008, 29(6): 1463-1468. [郭卫东, 程远月. 天然日光辐照下河口区CDOM的光化学降解. 环境科学, 2008, 29(6): 1463-1468.] |
| [11] |
Stedmon CA, Markager S, Tranvik L et al. Photochemical production of ammonium and transformation of dissolved organic matter in the Baltic Sea. Marine Chemistry, 2007, 104(3/4): 227-240. DOI:10.1016/j.marchem.2006.11.005 |
| [12] |
Zhang YL, Liu XH, Osburn CL et al. Photobleaching response of different sources of chromophoric dissolved organic matter exposed to natural solar radiation using absorption and excitation-emission matrix spectra. PLoS One, 2013, 8(10): e77515. DOI:10.1371/journal.pone.0077515 |
| [13] |
Gao HZ, Zepp RG. Factors influencing photoreactions of dissolved organic matter in a coastal river of the southeastern United States. Environmental Science and Technology, 1998, 32(19): 2940-2946. DOI:10.1021/es9803660 |
| [14] |
Hefner KH, Fisher JM, Ferry JL. A multifactor exploration of the photobleaching of Suwannee River dissolved organic matter across the freshwater/saltwater interface. Environmental Science and Technology, 2006, 40(12): 3717-3722. DOI:10.1021/es052513h |
| [15] |
Lalonde K, Mucci A, Ouellet A et al. Preservation of organic matter in sediments promoted by iron. Nature, 2012, 483(7388): 198-200. DOI:10.1038/nature10855 |
| [16] |
Vecchio RD, Blough NV. Photobleaching of chromophoric dissolved organic matter in natural waters:Kinetics and modeling. Marine Chemistry, 2002, 78(4): 231-253. DOI:10.1016/S0304-4203(02)00036-1 |
| [17] |
Keith DJ, Yoder JA, Freeman SA. Spatial and temporal distribution of coloured dissolved organic matter (CDOM) in Narragansett Bay, Rhode Island:Implications for phytoplankton in coastal waters. Estuarine Coastal and Shelf Science, 2002, 55(5): 705-717. DOI:10.1006/ecss.2001.0922 |
| [18] |
Helms JR, Stubbins A, Ritchie JD et al. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnology and Oceanography, 2007, 53(3): 955-969. DOI:10.4319/lo.2008.53.3.0955 |
| [19] |
Xiao YH, Timo SA, HelinÄ H et al. Contribution of ferric iron to light absorption by chromophoric dissolved organic matter. Limnology and Oceanography, 2013, 58(2): 653-662. DOI:10.4319/lo.2013.58.2.0653 |
| [20] |
Zhang YL, Zhang E, Yin Y et al. Characteristics and sources of chromophoric dissolved organic matter in lakes of the Yungui Plateau, China, differing in trophic state and altitude. Limnology and Oceanography, 2010, 55(6): 2645-2659. DOI:10.4319/lo.2010.55.6.2645 |
| [21] |
Mcknight DM, Boyer EW, Westerhoff PK et al. Spectrophotometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnology and Oceanography, 2001, 46(1): 38-48. DOI:10.4319/lo.2001.46.1.0038 |
| [22] |
Stedmon CA, Bro R. Characterizing dissolved organic matter fluorescence with parallel factor analysis:A tutorial. Limnology and Oceanography Methods, 2008, 6(11): 572-579. DOI:10.4319/lom.2008.6.572 |
| [23] |
Guo WD, Cheng YY. Photodegradation of chromophoric dissolved organic matter from Jiulong River Estuary under natural solar radiation. Environmental Science, 2008, 29(6): 1463-1468. [郭卫东, 程远月. 天然日光辐照下河口区CDOM的光化学降解. 环境科学, 2008, 29(6): 1463-1468.] |
| [24] |
Liu X, Zhang Y, Yin Y et al. Wind and submerged aquatic vegetation influence bio-optical properties in large shallow Lake Taihu, China. Journal of Geophysical Research Biogeosciences, 2013, 118(2): 713-727. DOI:10.1002/jgrg.20054 |
| [25] |
Osburn CL, Handsel LT, Mikan MP et al. Fluorescence tracking of dissolved and particulate organic matter quality in a river-dominated estuary. Environmental Science and Technology, 2012, 46(16): 8628-8636. DOI:10.1021/es3007723 |
| [26] |
Murphy KR, Ruiz GM, Dunsmuir WTM et al. Optimized parameters for fluorescence-based verification of ballast water exchange by ships. Environmental Science and Technology, 2006, 40(7): 2357-2362. DOI:10.1021/es0519381 |
| [27] |
Cory RM, Mcknight DM. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environmental Science and Technology, 2005, 39(21): 8142-8149. DOI:10.1021/es0506962 |
| [28] |
Cheng YY, Guo WD. Characterization of photobleaching of chromophoric dissolved organic matter in Xiamen bay by excitation emission matrix spectroscopy. Spectroscopy and Spectral Analysis, 2009, 29(4): 990-993. |
| [29] |
Sommaruga R. The role of solar UV radiation in the ecology of alpine lakes. J Photochem Photobiol B, 2001, 62(1/2): 35-42. DOI:10.1016/S1011-1344(01)00154-3 |
| [30] |
Kieber RJ, Zhou X, Mopper K. Formation of carbonyl compounds from UV-induced photodegradation of humic substances in natural waters:Fate of riverine carbon in the sea. Limnology and Oceanography, 1990, 35(7): 1503-1515. DOI:10.4319/lo.1990.35.7.1503 |
| [31] |
Yang DT, Chen WM, Wu SC et al. The effects of CDOC on the attenuation of close ultraviolet and blue light in Chaohu Lake and Longgan Lake. J Lake Sci, 2003, 15(3): 269-274. [杨顶田, 陈伟民, 吴生才等. 湖泊中有色可溶性物质对近紫外及蓝光衰减的影响. 湖泊科学, 2003, 15(3): 269-274. DOI:10.18307/2003.0312] |
| [32] |
Cory RM, Mcneill K, Cotner JP et al. Singlet oxygen in the coupled photochemical and biochemical oxidation of dissolved organic matter. Environmental Science and Technology, 2010, 44(10): 3683-3689. DOI:10.1021/es902989y |
| [33] |
Song XN, Yu T, Zhang Y et al. Distribution characterization and source analysis of dissolved organic matters in Taihu Lake using three dimensional fluorescence excitation-emission matrix. Journal of Environmental Sciences, 2010, 30(11): 2321-2331. [宋晓娜, 于涛, 张远等. 利用三维荧光技术分析太湖水体溶解性有机质的分布特征及来源. 环境科学学报, 2010, 30(11): 2321-2331.] |
 2018, Vol. 30
2018, Vol. 30 

