(2: 环境保护部南京环境科学研究所, 南京 210042)
(3: 浙江省环境监测中心, 杭州 310012)
(2: Nanjing Institute of Environmental Sciences, Ministry of Environmental Protection, Nanjing 210042, P. R. China)
(3: Zhejiang Environmental Monitoring Centre, Hangzhou 310012, P. R. China)
一氧化二氮(N2O)作为一种温室气体,其在大气中的浓度仅为二氧化碳(CO2)的1 ‰,但温室效应是CO2的296倍,所导致的全球性气候变化和生态环境问题已经成为21世纪人类面临的严重威胁[1].土壤、湿地系统和河流等被认为是N2O的重要自然排放源,近年来的研究表明底栖动物也是潜在的N2O排放源[2-3].底栖动物自身不能产生N2O,主要是通过两种途径产生N2O,一方面底栖动物肠道内的反硝化微生物经不完全反硝化作用产生N2O,这部分N2O约占底栖动物N2O释放量的30 % ~68 %,是底栖动物N2O排放的主要来源;另一方面底栖动物表面粘附的生物膜也是N2O释放的场所,硝化作用产生的N2O最高可达底栖动物释放量的1/3[4-5].底栖动物N2O排放能力与其栖息环境密切相关,当沉积物硝态氮浓度在25~50 μmol/L时,羽摇蚊幼虫(Chironomus plumosus larvae)释放N2O速率与温度呈正相关,当温度在4~10℃时,N2O释放速率随环境硝态氮浓度增加而升高,底栖动物释放N2O速率存在季节性变化,在夏季底栖动物释放N2O速率高而在冬季释放速率低[6-7].蓝藻暴发时溶解氧和硝态氮浓度下降,泥水界面的微环境发生明显改变,会促进湖泊水体向大气释放N2O,蓝藻堆积区常被认为是湖泊N2O排放的热点区域,而此时底栖动物N2O释放情况还不清楚[8-10].本实验采集太湖梅梁湾的蓝藻水华,以底栖动物羽摇蚊幼虫为研究对象,采用室内微宇宙实验,研究蓝藻对摇蚊幼虫排放N2O的影响,揭示富营养化湖泊底栖动物的环境效应,丰富湖泊氮转化过程.
1 材料与方法 1.1 实验材料2013年9月26日用彼得森采泥器采集太湖梅梁湾(31.42655°N, 120.20952°E)沉积物,并用64 μm的筛网过滤上覆水收集蓝藻水华,将采集的沉积物和蓝藻水华带回实验室备用.羽摇蚊幼虫购于天津宇峰养殖场,平均湿重约25 mg/ind.、长10 mm/ind.,为三龄和四龄的红色幼虫,为减少羽摇蚊幼虫肠道微生物对实验结果的影响,在实验前将羽摇蚊幼虫在清水中养殖3 d,每天换水.
1.2 实验设置将2 L混匀的沉积物分装到6个有机玻璃容器(直径0.2 m,高0.15 m),按照泥水比2:1沿壁采用静脉注射的方式缓慢加入2 mg/L硝态氮和2 mg/L铵态氮灭菌自来水,室温培养2周,每天更换上覆水.培养结束后,向每个有机容器中引入20条大小一致的羽摇蚊幼虫(密度为1000 ind./m2),将6个容器随机分成2组,每组3个平行,一组为不加藻的对照组,另一组为添加蓝藻的处理组,添加冻干蓝藻的浓度则由梅梁湾中上覆水叶绿素a浓度(500 μg/L)换算而来[11-12].所有的微宇宙在25℃避光培养2周,用0.5 mm的筛网收集存活的羽摇蚊幼虫,对照组和实验组的羽摇蚊幼虫存活率分别为95 %和85 %.
1.3 羽摇蚊幼虫释放N2O通量的测定羽摇蚊幼虫活体和肠道N2O释放通量参考Stief的方法进行测定[2].各组分别取10条羽摇蚊幼虫,用无菌水清洗羽摇蚊幼虫表面3次,放于含200 μl NaNO3(0.5 mmol/L)的2 ml西林瓶中,每个西林瓶内放置一条羽摇蚊幼虫,丁基橡胶密封瓶口后21℃避光培养2 h,收集西林瓶顶空气体测定N2O含量,作为羽摇蚊幼虫活体排放N2O通量.将10个羽摇蚊幼虫的肠道放在含0.5 ml的Tris-HCl(0.1 mol/L,pH 7.5)缓冲液的2 ml西林瓶,加入100 μl含有葡萄糖、NaNO3和链霉素的溶液,使其终浓度分别为2、2和0.4 mmol/L,用丁基橡胶密封,30℃避光培养2 h,测定西林瓶内N2O含量.
1.4 羽摇蚊幼虫肠道细菌和反硝化菌的测定分别取培养前(G0)、无藻(GN)和加藻(GA)培养羽摇蚊幼虫10条,解剖获得30个肠道(主要为中肠和后肠),将每个处理组的10个肠道合并为1个肠道样品,参照Horn等[13]的方法提取3个羽摇蚊幼虫肠道DNA.将提取的肠道DNA用特异性引物27F/533R对细菌16S rRNA基因进行PCR扩增,纯化后使用454 FLX Titanium完成测序;采用引物cd3af(GTSAACGTSAAGGARACSGG)/r3cd(GASTTCGGRTGSGTCTTGA)[14]对nirS基因进行PCR扩增,纯化后经载体pMD-19T(Takara)克隆到E. coil JM 109,得到G0、GN、GA三个克隆文库,将阳性克隆送华大基因公司测序.
1.5 数据分析方差分析(analysis of variance, ANOVA)在SPSS 18.0软件上进行. 454测序结果采用Mothur软件进行降噪处理,去掉长度小于300 bp、模糊碱基和引物碱基错配2个以上、质量分数低于25、单碱基重复超过6个不确定碱基的序列以及嵌合体序列.将得到的高质量序列经UCLUST按照97 %的相似度进行可操作分类单元(operational taxonomic unit, OTU)分类,选取每个OTU中数量最多的序列作为代表性序列,将代表性序列上传至Ribosomal Database Project(RDP)进行物种注释,参考数据库为Silva的16S rRNA基因数据库,置信度为0.8,为减少测序深度的影响,将每个样品的序列统一至3068条,再分析样品的群落多样性指数.将获得的nirS基因序列去除载体、引物及可能存在的嵌合体后,nirS基因序列长度约为420 bp,采用Mothur软件计算克隆文库的OTU和多样性指数(Shannon-Wiener指数、ACE指数、Chao1指数). Shannon-Wiener指数的计算按照H′=-∑(ni/N)(ln ni/N),其中ni为样品中属于第i种的个体数目,N为样品总个体数;ACE指数计算公式为:SACE=Sabund+Srare/CACE+n1/CACE,其中CACE=n1/Nrare,
蓝藻水华显著降低了羽摇蚊幼虫N2O释放通量(P < 0.01),羽摇蚊幼虫N2O释放通量降低了60 %.处理组和对照组羽摇蚊幼虫肠道N2O释放通量分别为12.0和25.5 pmol/(ind. ·h),分别占活体释放通量的77 %和66 %,蓝藻水华使羽摇蚊幼虫肠道N2O释放通量降低52.9 %,表明肠道是羽摇蚊幼虫释放N2O的主要场所,蓝藻水华减缓了羽摇蚊幼虫N2O的释放(图 1).

|
图 1 蓝藻水华对羽摇蚊幼虫N2O释放的影响(n=10) Fig.1 The influence of cyanobacterial bloom on N2O emission efflux from C. plumosus larvae and the gut(n=10) |
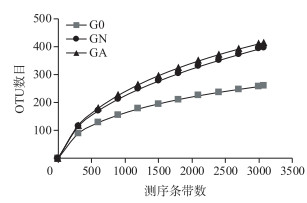
利用高通量测序技术,经序列过滤和去除嵌合体后,3个肠道样品共得到11976条高质量序列,长度分布在300~535 bp之间,其中以序列499 bp居多. 97 %的相似度下共获得768个OTU,G0、GN和GA三个样品的OTU数目分别为256、395和412,经2周的培养,羽摇蚊幼虫肠道OTU数目均大幅增加,其中GA处理OTU数目最高,预示更多的细菌种类(表 1). 3个样品的稀释曲线在97 %的相似度下趋于平缓,达到饱和,说明统一测序深度至3068条时,3个样品获取的细菌信息基本能反映肠道细菌的群落组成(图 2).
|
图 2 OTU稀释曲线 Fig.2 Rarefaction curves of OTUs |
| 表 1 羽摇蚊幼虫肠道细菌多样性指数* Tab.1 Richness and biodiversity indexes in gut samples from C. plumosus larvae |
通过Chao1、ACE以及Shannon-Wiener指数来表征羽摇蚊幼虫肠道细菌多样性,结果表明,与培养前相比,经2周培养的羽摇蚊幼虫肠道细菌多样性均有所增加,但蓝藻水华处理组的细菌多样性低于对照组,表明蓝藻水华能降低羽摇蚊幼虫肠道内细菌多样性(表 1).
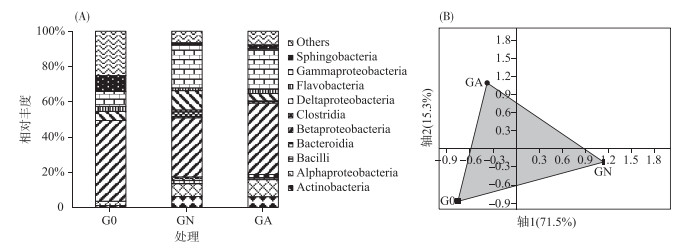
培养前羽摇蚊幼虫肠道内细菌主要由鞘脂杆菌、β-变形菌、δ-变形菌和γ-变形菌组成,约占细菌总数的68.5 % (图 3A).经2周培养后,不同处理羽摇蚊幼虫肠道细菌群落结构发生明显变化,对照组羽摇蚊幼虫肠道以放线菌、α-变形菌、β-变形菌、δ-变形菌和γ-变形菌为主,其相对丰度达到了82.3 %,而处理组羽摇蚊幼虫肠道则以放线菌、α-变形菌、β-变形菌和γ-变形菌为主,占总细菌的79.1 %.蓝藻能使羽摇蚊幼虫肠道中β-变形菌相对丰度增加21 %,δ-变形菌相对丰度减少62 %.通过PCA分析(图 3B),发现了类似的变化规律,3个样品不能聚在一起,前两轴解释了大约87 %的细菌群落差异,表明蓝藻水华的存在可显著改变羽摇蚊幼虫肠道细菌群落结构.
|
图 3 不同处理的羽摇蚊幼虫细菌群落组成(A)和PCA分析(B) Fig.3 The relative abundance of bacterial communities with class(A) and PCA plot of bacterial communities(B) in gut samples from C. plumosus larvae |
羽摇蚊幼虫N2O释放能力与其肠道反硝化微生物有关,为了探究蓝藻对羽摇蚊幼虫肠道反硝化微生物群落的影响,以nirS基因为反硝化菌标记物,分别对培养前、不加藻处理和加藻处理的羽摇蚊幼虫肠道反硝化菌建立的3个克隆文库,从每个文库随机选取16~44个克隆子进行测序,得到85个有效克隆(表 2),克隆文库的盖度在50 % ~74 %之间,基本覆盖优势的nirS型反硝化菌.通过Chao 1、ACE和Shannon-Wiener指数分析,发现经2周培养,不同处理的反硝化菌多样性均有所升高,与对照组相比,蓝藻的添加导致羽摇蚊幼虫肠道反硝化菌多样性的显著增加,表明蓝藻水华能增加羽摇蚊幼虫肠道反硝化菌多样性.
| 表 2 羽摇蚊幼虫肠道反硝化菌多样性 Tab.2 Richness and biodiversity indexes of nirS gene in gut samples from C. plumosus larvae |
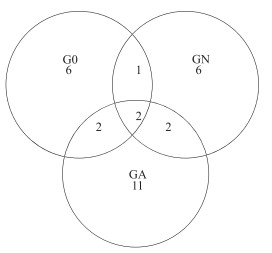
3个克隆文库获得的85个克隆子可分为30个OTU,大多数的OTU(93 %)分布在1个或2个文库中,只有2个OTU分布在所有的文库中,加藻处理组的特异性OTU最多(图 4),表明蓝藻暴发时羽摇蚊幼虫肠道内出现了数目较多的特有的nirS基因型反硝化菌表型.
|
图 4 不同处理羽摇蚊幼虫肠道nirS基因Veen图 Fig.4 Shared OTU of nirS gene in three gut samples from C. plumosus larvae |
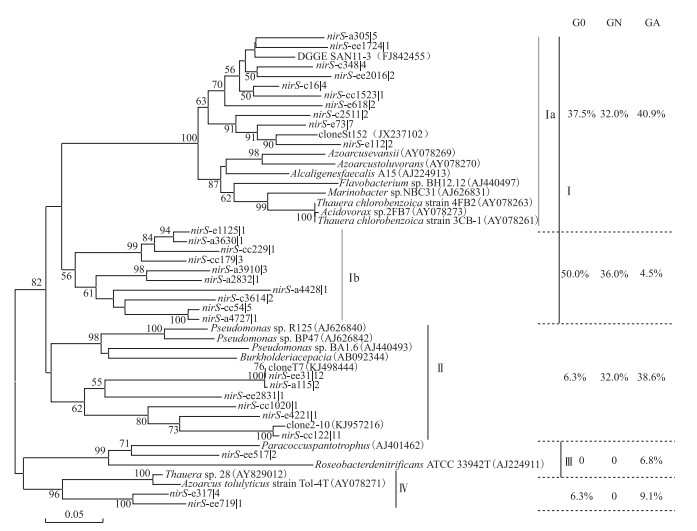
所有的nirS基因序列可分为4个簇,培养前的羽摇蚊幼虫肠道nirS序列以Ⅰa和Ⅰb亚簇为主,相对丰度达到87.5 % (图 5).经2周培养后,对照组nirS型反硝化菌为Ⅰa、Ⅰb亚簇和Ⅱ簇(共计100 %),而处理组则以Ⅰa亚簇和Ⅱ簇为主,相对丰度为79.5 %,Ⅰb亚簇相对丰度减少了87.5 %,并出现了特有的Ⅲ簇序列(6.8 %),表明蓝藻水华可改变羽摇蚊幼虫肠道反硝化菌群落结构.
|
图 5 羽摇蚊幼虫肠道nirS基因的系统发育树 Fig.5 Phylogenetic analysis of nirS gene in gut samples from C. plumosus larvae(Bootstrap values greater than 50 % are shown, The scale bar indicates the number of changes per sequence position) |
随着城市化和工业化水平的提高,人类活动对湖泊的影响也越来越大,湖泊的富营养化程度加剧,促进湖泊N2O的排放,使湖泊成为继稻田、湿地等传统N2O排放源之外的另一个重要的N2O释放源[15-17].蓝藻能直接或间接地影响湖泊N2O的排放,蓝藻通过光合作用产生氧气加速水体的硝化作用,从而增强耦合的硝化反硝化作用,促进N2O的释放,所以蓝藻堆积的区域是N2O释放的热点区域[18]. Wang等[19]研究发现富营养化程度较为严重的梅梁湾夏季时水气界面N2O释放通量为5.28~85.8 μg N2O/(m2 ·h),Liu等[20]对莫愁湖、团结湖和大明湖蓝藻堆积区发现水气界面N2O释放通量为3.9~11.3 μg N2O/(m2 ·h).在藻型湖泊中底栖动物以收集者为主,例如耐污的双壳纲类紫贻贝(Mytilus edulis)和摇蚊属类羽摇蚊幼虫等,这类底栖动物N2O释放量通常在100~700 pmol N2O/(ind. ·h)[2].本研究中,无藻环境下,羽摇蚊幼虫释放N2O的通量为19.25 pmol N2O/(ind. ·h),仅为丹麦Großer Binnensee湖羽摇蚊幼虫N2O释放通量的22.8 %,这可能与不同的湖泊环境有关[21].根据太湖梅梁湾羽摇蚊幼虫最高密度1280 ind./m2计算[12, 22],羽摇蚊幼虫N2O释放通量可达1.08 μg N2O/(m2 ·h),蓝藻暴发时羽摇蚊幼虫N2O的释放通量下降到0.65 μg N2O/(m2 ·h),约占梅梁湾湖区泥水界面N2O释放通量的0.75 %,而向大气排放量可减少0.5 % ~8.1 %,表明蓝藻虽然促进湖泊水体向大气释放N2O温室气体,但作为N2O潜在释放源的羽摇蚊幼虫的贡献则有所下降[8, 23].
含大量微生物的沉积物颗粒以及碎屑通过吞食作用进入底栖动物肠道,发生反硝化作用,将无机氮转化成N2O或N2,向环境中排放温室气体N2O,底栖动物肠道N2O释放通量约占活体的65 % ~78 %,本研究中羽摇蚊幼虫肠道N2O释放通量约占活体的77 %,与前人的研究结果一致[2, 5].蓝藻水华存在时沉积物和上覆水的微环境发生改变,进而导致羽摇蚊幼虫肠道环境也发生变化,研究表明,当上覆水硝态氮浓度低于15.5 mg/L时,羽摇蚊幼虫肠道硝态氮浓度与上覆水硝态氮浓度呈较好的正相关[24],因此,高浓度硝态氮会导致反硝化过程中释放更多的N2O,尤其在富含有机质的环境且没有明显的硝态氮输入时,会增加C:NO3-的比例,降低催化反硝化过程中一系列酶活性以及NO3-扩散到反硝化位点的速率,从而减少反硝化过程中N2O:N2的比例[25].
采用高通量测序和克隆文库技术显示,蓝藻水华存在时羽摇蚊幼虫肠道内细菌以及nirS型反硝化菌群落结构发生明显的变化.蓝藻暴发时羽摇蚊幼虫肠道细菌多样性降低,细肠道内β-变形菌相对丰度增加到40.6 %,δ-变形菌相对丰度降低到4.1 %,可能与羽摇蚊幼虫摄食模式及肠道独特的微环境有关[26-27].大多数情况下,羽摇蚊幼虫食物组成以有机碎屑为主,而在藻类大量存在时,其食物中硅藻和绿藻比例上升,有机碎屑比率下降,这对肠道微生物群落结构也会起到一定的影响[28].在自然环境中大约1/3的反硝化菌含有nirS或nirK基因,本研究中获得的nirS基因序列与已知的反硝化细菌基因序列具有较高的相似性,主要属于β-变形菌,其余为α-和γ-变形菌,通常存在于羽摇蚊幼虫、白蚁肠道和贝类的肠道内[3, 29-30].加藻处理组羽摇蚊幼虫肠道内nirS型反硝化菌的表型具有更广的进化范围(Ⅰ~Ⅳ簇),主要以Ⅰa(β-变形菌)和Ⅱ簇(γ-变形菌)为主,代表性反硝化菌分别为陶厄氏菌(Thauera)和假单胞菌(Pseudomonas),能还原硝酸盐[31]. Ⅰb亚簇(β-变形菌)急剧降低至4.5 %,其中代表性菌为丛毛单胞菌(Comamonas),其反硝化能力还有待进一步研究[32]. Ⅲ簇序列(6.8 %)仅在有藻微宇宙羽摇蚊幼虫肠道内检测到,这一簇内代表性菌为α-变形菌的Paracoccus,这类反硝化菌在好氧和厌氧环境下均能进行反硝化作用[33].
4 结论蓝藻水华存在时羽摇蚊幼虫N2O排放通量减少60 %,改变羽摇蚊幼虫肠道内细菌和反硝化菌群落结构,主要结论如下:
1) 羽摇蚊幼虫肠道是其N2O排放的主要场所,蓝藻的堆积可降低羽摇蚊幼虫N2O排放通量.
2) 蓝藻水华的存在能降低羽摇蚊幼虫肠道内细菌的多样性,增加羽摇蚊幼虫肠道内β-变形菌相对丰度,降低δ-变形菌相对丰度,改变肠道内细菌群落结构;增加反硝化菌多样性,出现较多的nirS型反硝化菌的表型,强化反硝化作用.
未来应加强蓝藻水华对底栖动物肠道微生物群落结构和功能的野外研究,探索不同生境下蓝藻对底栖动物释放N2O潜力的影响,进一步丰富湖泊生态学的内涵.
| [1] |
Jung MY, Well R, Min D et al. Isotopic signatures of N2O produced by ammonia-oxidizing archaea from soils. The International Society for Microbial Ecology Journa, 2014, 8: 1115-1125. DOI:10.1038/ismej.2013.205 |
| [2] |
Stief P, Poulsen M, Nielsen LP et al. Nitrous oxide emission by aquatic macrofauna. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(11): 4296-4300. DOI:10.1073/pnas.0808228106 |
| [3] |
Poulsen M, Kofoed MV, Larsen LH et al. Chironomus plumosus larvae increase fluxes of denitrification products and diversity of nitrate-reducing bacteria in freshwater sediment. Systematic and Applied Microbiology, 2014, 37(1): 51-59. DOI:10.1016/j.syapm.2013.07.006 |
| [4] |
Heisterkamp IM, Schramm A, Larsen LH et al. Shell biofilm-associated nitrous oxide production in marine molluscs:processes, precursors and relative importance. Environmental Microbiology, 2013, 15(7): 1943-1955. DOI:10.1111/j.1462-2920.2012.02823.x |
| [5] |
Heisterkamp IM, Schramm A, de Beer D et al. Nitrous oxide production associated with coastal marine invertebrates. Marine Ecology Progress Series, 2010, 415: 1-9. DOI:10.3354/meps08727 |
| [6] |
Stief P, Schramm A. Regulation of nitrous oxide emission associated with benthic invertebrates. Freshwater Biology, 2010, 55: 1647-1657. DOI:10.1111/j.1365-2427.2010.02398.x |
| [7] |
Stief P, Polerecky L, Poulsen M et al. Control of nitrous oxide emission from Chironomus plumosus larvae by nitrate and temperature. Limnology and Oceanography, 2010, 55(2): 872-884. DOI:10.4319/lo.2010.55.2.0872 |
| [8] |
Chen XF, Jiang HY, Sun X et al. Nitrification and denitrification by algae-attached and free-living microorganisms during a cyanobacterial bloom in Lake Taihu, a shallow Eutrophic Lake in China. Biogeochemistry, 2016, 131(1/2): 135-146. DOI:10.1007/s10533-016-0271-z |
| [9] |
Wang HJ, Yang LY, Wang WD et al. Nitrous oxide(N2O) fluxes and their relationships with water-sediment characteristics in a hyper-eutrophic shallow lake, China. Journal of Geophysical Research, 2007, 112(G1): 129-137. DOI:10.1029/2005JG000129 |
| [10] |
Xia Y, Li Y, Li X et al. Diurnal pattern in nitrous oxide emissions from a sewage-enriched river. Chemosphere, 2013, 92(4): 421-428. DOI:10.1016/j.chemosphere.2013.01.038 |
| [11] |
Wang HJ, Wang WD, Yin CQ et al. Littoral zones as the "hotspots" of nitrous oxide(N2O) emission in a hyper-eutrophic lake in China. Atmospheric Environment, 2006, 40(28): 5522-5527. DOI:10.1016/j.atmosenv.2006.05.032 |
| [12] |
Cai YJ, Gong ZJ, Qin BQ. Influences of habitat type and environmental variables on benthic macroinvertebrate communities in a large shallow subtropical lake(Lake Taihu, China). Annales de Limnologie-International Journal of Limnology, 2010, 47(1): 85-95. DOI:10.1051/limn/2010028 |
| [13] |
Horn MA, Drake HL, Schramm A. Nitrous oxide reductase genes(nosZ) of denitrifying microbial populations in soil and the earthworm gut are phylogenetically similar. Applied and Environmental Microbiology, 2006, 72(2): 1019-1026. DOI:10.1128/AEM.72.2.1019-1026.2006 |
| [14] |
Michotey V, Mejean V, Bonin P. Comparison of methods for quantification of cytochrome cd(1)-denitrifying bacteria in environmental marine samples. Applied and Environmental Microbiology, 2000, 66(4): 1564-1571. DOI:10.1128/AEM.66.4.1564-1571.2000 |
| [15] |
Saarenheimo J, Rissanen A, Arvola L et al. Genetic and environmental controls on nitrous oxide accumulation in lakes. PLoS One, 2015, 10(3): e0121201. DOI:10.1371/journal.pone.0121201 |
| [16] |
Musenze RS, Grinham A, Werner U et al. Assessing the spatial and temporal variability of diffusive methane and nitrous oxide emissions from subtropical freshwater reservoirs. Environmental Science Technology, 2014, 48(24): 14499-14507. DOI:10.1021/es505324h |
| [17] |
Wang S, Liu C, Yeager KM et al. The spatial distribution and emission of nitrous oxide(N2O) in a large eutrophic lake in eastern China:Anthropogenic effects. Science of the Total Environment, 2009, 407(10): 3330-3337. DOI:10.1016/j.scitotenv.2008.10.037 |
| [18] |
Yan X, Xu X, Wang M et al. Climate warming and cyanobacteria blooms:Looks at their relationships from a new perspective. Water Research, 2017, 125: 449-457. DOI:10.1016/j.watres.2017.09.008 |
| [19] |
Wang DQ, Chen ZL, Wang J et al. Summer-time denitrification and nitrous oxide exchange in the intertidal zone of the Yangtze Estuary. Estuarine, Coastal and Shelf Science, 2007, 73(1/2): 43-53. DOI:10.1016/j.ecss.2006.11.002 |
| [20] |
Liu YS, Zhu RB, Ma DW et al. Temporal and spatial variations of nitrous oxide fluxes from the littoral zones of three alga-rich lakes in coastal Antarctica. Atmospheric Environment, 2011, 45(7): 1464-1475. DOI:10.1016/j.atmosenv.2010.12.017 |
| [21] |
Stief P, Schramm A, Altmann D et al. Temporal variation of nitrification rates in experimental freshwater sediments enriched with ammonia or nitrite. FEMS Microbiol Ecology, 2003, 46(1): 63-71. DOI:10.1016/S0168-6496(03)00193-4 |
| [22] |
Cai YJ, Gong ZJ, Qin BQ. Community structure and diversity of macrozoobenthos in Lake Taihu, a large shallow eutrophic lake in China. Biodiversity Science, 2010, 10(1): 50-59. [蔡永久, 龚志军, 秦伯强. 太湖大型底栖动物群落结构及多样性. 生物多样性, 2010, 10(1): 50-59. DOI:10.3724/SP.J.1003.2010.050] |
| [23] |
Zhong JC, Fan CX, Liu GF et al. Seasonal variation of potential denitrification rates of surface sediment from Meiliang Bay, Taihu Lake, China. Journal of Environmental Sciences, 2010, 22(7): 961-967. DOI:10.1016/S1001-0742(09)60205-9 |
| [24] |
Stief P, Polerecky L, Poulsen M et al. Control of nitrous oxide emission from Chironomus plumosus larvae by nitrate and temperature. Limnology and Oceanography, 2010, 55(2): 872-884. DOI:10.4319/lo.2010.55.2.0872 |
| [25] |
Saggar S, Jha N, Deslippe J et al. Denitrification and N2O:N2 production in temperate grasslands:processes, measurements, modelling and mitigating negative impacts. Science of the Total Environment, 2013, 465: 173-195. DOI:10.1016/j.scitotenv.2012.11.050 |
| [26] |
Stief P, Beer D. Probing the microenvironment of freshwater sediment macrofauna:implications of deposit-feeding and bioirrigation for nitrogen cycling. Limnology and Oceanography, 2006, 51(6): 2538-2548. DOI:10.4319/lo.2006.51.6.2538 |
| [27] |
Stief P. Stimulation of microbial nitrogen cycling in aquatic ecosystems by benthic macrofauna:Mechanisms and environmental implications. Biogeosciences, 2013, 10(12): 7829-7846. DOI:10.5194/bgd-10-11785-2013 |
| [28] |
Liu XQ. Food composition and food webs of zoobenthos in Yangtze lakes[Dissertation]. Wuhan: Institute of Hydrobiology, Chinese Academy of Sciences, 2006: 67-80. [刘学勤. 湖泊底栖动物食物组成与食物网研究[学位论文]. 武汉: 中国科学院水生生物研究所, 2006: 67-80. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1616223 ]
|
| [29] |
Harter J, Krause HM, Schuettler S et al. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. The International Society for Microbial Ecology Journal, 2014, 8(3): 660-674. |
| [30] |
Ngugi DK, Brune A. Nitrate reduction, nitrous oxide, and anaerobic ammonia oxidation to nitrite in the gut of soil-feeding termites(Cubitermes and Ophiotermes spp.). Environmental Microbiology, 2012, 14(4): 860-871. DOI:10.1111/j.1462-2920.2011.02648.x |
| [31] |
Liang LH, Zuo JE. Denitrifying functional genes-the molecular marker for detection of denitrifying community structure. Microbiology, 2009, 36(4): 627-633. [梁丽华, 左剑恶. 反硝化功能基因——检测反硝化菌种群结构的分子标记. 微生物学报, 2009, 36(4): 627-633.] |
| [32] |
Bellini MI, Gutierrez L, Tarlera S et al. Isolation and functional analysis of denitrifiers in an aquifer with high potential for denitrification. Systematic and Applied Microbiology, 2013, 36(7): 505-516. DOI:10.1016/j.syapm.2013.07.001 |
| [33] |
Xiao JJ, Guo P, Huo WJ et al. Application of denitrifying microbes to wastewater denitrification. Environmental Science Technology, 2009, 32(12): 97-102. [肖晶晶, 郭萍, 霍炜洁等. 反硝化微生物在污水脱氮中的研究及应用进展. 环境科学与技术, 2009, 32(12): 97-102. DOI:10.3969/j.issn.1003-6504.2009.12.022] |
 2018, Vol. 30
2018, Vol. 30 

