(2: 中国科学院大学, 北京 100049)
(2: University of Chinese Academy of Sciences, Beijing 100049, P. R. China)
近年来,随着工业的发展和人类活动加剧,越来越多的外源性有机碳进入到水体生态系统中,成为湖泊碳库的重要组成部分. 1970s, Fisher等[1]开始提出来自外源的碳补贴能主导生态系统的碳收支,并强烈影响生态系统动力学,Simon等[2]认为内陆水体中外源性有机碳实质上补充了内源性有机碳储存.而近年来亦有研究表明,一些寡营养或高腐殖质含量的湖泊中,浮游生物体生长尤其依赖于外源有机碳[3-6].然而外源输入的DOM大多是高分子量的腐殖质类物质,传统观点认为其生物可利用性较低[7].所以外源DOM究竟以何种途径进入湖泊碳循环过程这一问题一直以来都是湖泊生态学研究中的重要课题.尽管外源DOM大多难以被水生生物直接吸收利用,但值得关注的是,这些高分子量的DOM中通常含有许多可吸收太阳光的显色基团,因而具有显著的光化学活性,容易发生光化学降解反应,产生小分子物质[8-9],进而增加其生物可利用性[10-12],并影响整个水生生态系统的物质循环和能量流动.
DOM的光降解效应很大程度取决于光照条件以及DOM的结构组成[13-14].迄今,关于湖泊外源DOM的光化学降解过程研究尚不够深入,这主要是由于外源DOM结构的多样性和复杂性,使得人们对其化学组成和结构缺乏分子水平上的认识.基于上述分析,如何进一步认识外源DOM的组成、结构以及光化学降解规律值得深入研究.三维荧光光谱-平行因子分析法(3D EEM-PARAFAC)作为一种可准确地识别样品中荧光峰的个数及荧光强度等信息的手段,近年来被广泛用于天然水体中的溶解性有机质特性研究,包括DOM的来源识别[15-16]、DOM的时间(光降解)、空间动态变化[14, 17-19]等等.
外源DOM的主要组成部分为腐殖质,可通过地下水和地表水循环进入湖泊[20-21].本研究以高山湖泊天才湖周边土壤中提取的天然腐殖酸作为外源DOM的代表物,通过室内模拟腐殖酸光降解实验,探明外源DOM光化学降解规律与降解产物信息,为开展外源碳的归趋研究增加科学积累.
1 材料与方法 1.1 腐殖质来源介绍云南老君山天才湖(26°37′~26°39′N, 99°42′~99°44′E,海拔3898 m)地处偏远地区,极少受到人类活动的干扰,湖水主要由降水、季节性溪流补给,外源碳输入量较高,属腐殖质湖泊.采集天才湖岸边5 m范围内的表层(3 cm以上)土壤,冷冻干燥备用.
1.2 研究方法根据国际腐殖酸协会(IHSS)提供的标准方法提取腐殖酸.步骤如下:称取100克干燥研磨后的土壤,过80目筛网并溶解于1 L的蒸馏水中,用1 mol/L的HCl调节pH为1~2,震荡1 h后低速离心;用1 mol/L的NaOH溶解下层沉淀后在N2氛围下震荡4 h以上并静置过夜,取上清液用6 mol/L的HCl调节pH为1,静置12 h后离心;在N2氛围下,用0.1 mol/L的KOH溶解沉淀物并加入KCl使得K+浓度为0.3 mol/L,高速离心后弃去沉淀物,用6 mol/L的HCl调节上清液pH为1,沉淀析出腐殖酸后再静置12~16 h,离心取沉淀加入0.1 mol/L的HCl和0.3 mol/L的HF混合液,重复震荡离心2~3次,最后将沉淀转移至透析袋中,用AgNO3检测至无氯离子析出,冷冻干燥得到纯化的腐殖酸.
将提取的腐殖酸用Milli-Q水配制成10 mg/L的溶液,体积为10 L,加入0.5 mol/L的NaOH调节pH值为8左右.照射实验共设置3组,包括避光对照组、20 W和40 W紫外灯照射组(光照强度分别为75和117 μW/cm2),每组设3个平行,实验进行54天,每隔2天取1次水样,测定溶液的紫外—可见光吸收光谱和三维荧光光谱.为了将微生物对实验结果的影响降至最小,实验中配制腐殖酸溶液选用的是无菌水,实验过程中反应容器使用保鲜膜覆盖,此外紫外灯也具有一定的杀菌作用.
1.3 分析方法 1.3.1 紫外-可见光谱法使用Lambda 35(Perkin Elmer)紫外可见分光光度计对样品进行全波段(200~800 nm)扫描,以Milli-Q水样为参照,得到各个波长下的吸光度值.
某一波长λ下的吸收系数aλ通常可以用来表示有色溶解性有机物(CDOM)的相对浓度[22],本文选取440 nm下的吸收系数,根据公式(1)和(2)可以计算一定波长下的吸光系数aλ:
| $ a_{\lambda^{\prime}}=2.303 D_{\lambda} / r $ | (1) |
式中,aλ'为波长λ下未校正的吸收系数(m-1);Dλ为吸光度;r为光程路径(0.01 m).由于过滤清液可能残留细小颗粒会引起散射,为此作如下散射效应订正[16]:
| $ a_{\lambda}=a_{\lambda^{\prime}}-a_{750} \lambda / 750 $ | (2) |
E4/E6即紫外可见光谱在465和665 nm波长处吸收系数的比值,可以用来表征CDOM溶液在光降解过程中的芳香化程度和相对分子量的变化,其值越低,溶液的相对分子质量越小、芳香化程度越高[23].
| $ \mathrm{E}_{4} / \mathrm{E}_{6}=a_{465} / a_{665} $ | (3) |
式中,a465和a665分别为波长465和665 nm处的吸收系数(m-1).
光谱斜率系数SR能够反映出CDOM的平均分子量的大小[24],本文采用Helms等[24]提出的对275~295 nm与350~400 nm两个波段进行非线性拟合得到的光谱斜率比值SR.随着SR的增加,溶液相对分子量不断减小.
| $ S_{\mathrm{R}}=S_{(275-295)} / S_{(350-400)} $ | (4) |
式中,S(275-295)和S(350-400)为波长275~295和350~400 nm下的光谱斜率(nm-1).
1.3.2 三维荧光光谱-平行因子分析法采用日立F-7000荧光光谱仪(Hitachi High-Technologies)测定EEMs数据,仪器基本参数设置如下:PMT电压700 V,激发波长(Ex)200~450 nm,步长5 nm,发射波长(Em)250~600 nm,步长为1 nm,响应时间为0.1 s,扫描速度为2400 nm/min.以Milli-Q超纯水为空白样.将测得的三维荧光数据输入到MATLAB R2008a软件中,并建立EEM-PARAFAC模型.
采用准一级降解动力学方程计算各个PARAFAC组分的降解速率[25]:
| $ \ln C / C_{0}=-k \cdot t+A $ | (5) |
式中,C为某一光照时间PARAFAC组分的荧光强度(RU);C0为该组分的初始荧光强度(RU);t为光照时间(d);k为拟一级反应动力学常数(d-1),k值越大表示光化学降解率越快;A为常数.
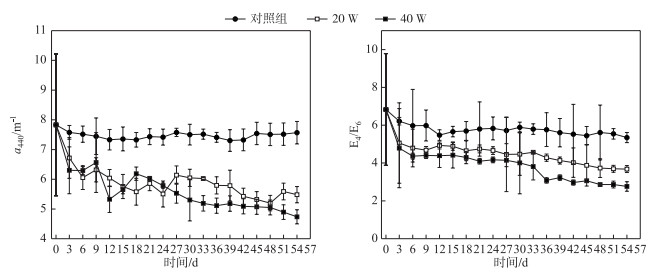
2 结果与分析 2.1 紫外-可见光吸收光谱分析 2.1.1 不同光照强度下吸收系数变化腐殖酸成分复杂, 浓度确定较为困难, 常见方法是用355、375和440 nm等波长处的吸收系数来表示其浓度.本文采用440 nm波长处的吸收系数a440表示,a440越大, 说明腐殖酸浓度越高.对照组、20 W组和40 W组的a440最终的下降速率分别为6.73 % (7.83~7.57 m-1)、33.63 % (7.83~5.48 m-1)和39.52 % (7.83~4.74 m-1)(图 1).对照组a440变化幅度最小,表明对照组腐殖酸溶液浓度下降幅度较小. 20 W组和40 W组溶液浓度不断下降,说明腐殖酸溶液在紫外光照射下发生了光降解作用,且随着光照强度的增加,腐殖酸的光降解率也随之增加.对吸收系数进行线性回归,计算不同光照强度下的降解动力学常数Kapp.对照组、20 W组和40 W组Kapp分别为0.0012、0.0255和0.038 d-1.这一结果进一步证明了光照强度对腐殖酸光降解行为有明显的影响,光照强度越大则腐殖酸降解速率就越大.
|
图 1 不同光照强度下吸收系数a440和E4/E6的变化 Fig.1 Variations of a440 and E4/E6 under different UV irradiation intensities |
3个组E4/E6值都在不断的下降,对照组、20 W组和40 W组的E4/E6值经实验处理后从6.83分别降至5.35、3.68和2.76,其值的变化幅度为:对照组 < 20 W组 < 40 W组,表明光照破坏了腐殖酸的结构,产生了小分子物质,而且光照强度越强,溶液的芳香化程度就越高,腐殖酸的降解程度也越大(图 1).
2.1.3 不同光照强度下光谱斜率SR变化3个组SR值随时间的变化不明显,对照组SR值在0.96~1.04之间,20 W组SR值在0.97~1.08之间,40 W组SR值在0.97~1.11之间.对a440、E4/E6和SR进行相关性分析,由表 1可知,a440与E4/E6呈显著正相关关系(P < 0.01),SR与a440、E4/E6均呈显著负相关关系(P < 0.01). 图 2显示SR与a440呈显著负相关关系. SR值的不断增加可以表明腐殖酸分子量在紫外辐射下不断减少,并生成了小分子物质,该结果与E4/E6的结果互相印证.
| 表 1 a440、E4/E6和SR之间的相关性 Tab. 1 The correlation between a440, E4/E6 and SR |

|
图 2 不同光照强度下SR与a440的相关关系 Fig.2 The correlation between SR and a440 under different UV irradiation intensities |
经EEM-PARAFAC模型运算,本研究共解析出5个组分,图 3展示了5个组分的三维荧光光谱图和其激发、发射特征图. 5个组分分别属于类腐殖质和类蛋白质两大类,其中C1(Component1;Em=430 nm/Ex < 240 nm)属UV类腐殖质[18, 26-27];C2(Component2;Em=496 nm/Ex=265 nm)属UVA类腐殖质[26-28];C3(Component3;Em=330 nm/Ex=275 nm)属类色氨酸[26-28],荧光特征类似于游离的色氨酸;C4(Component4;Em=432 nm/Ex=275 nm/335 nm)属未知或UVC类腐殖质[26, 29];C5(Component5;Em=310 nm/Ex=245 nm)属类络氨酸[30-31],荧光特征类似于游离的络氨酸.腐殖酸溶液中C1~C5的未降解初始荧光强度分别为1.68、0.56、0.66、0.54和0.49 RU,各组分对总荧光强度贡献比例分别为42.48 %、15.13 %、16.28 %、13.77 %和12.34 %,说明腐殖酸的荧光贡献中以UV类腐殖质组分C1为主,类色氨酸、UVA类腐殖质、UVC类腐殖质、类络氨酸组分浓度贡献接近.

|
图 3 C1~C5组分三维荧光图和对应的激发、发射特征 Fig.3 The PARAFAC model and the spectral shapes of excitation and emission of C1-C5 |
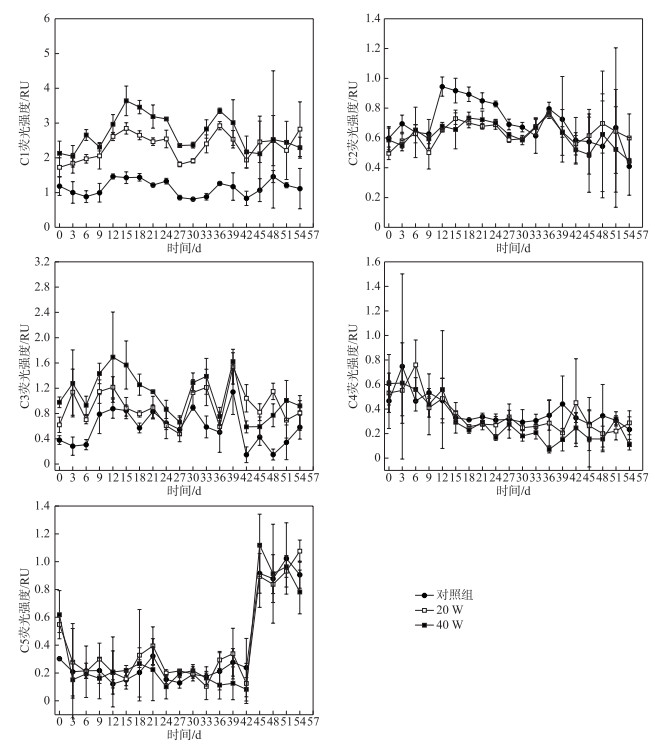
图 4显示了光降解过程中5个组分平均荧光强度随时间变化幅度差异较大,C1、C2和C3组分荧光强度先增加后减少,C1组分从第15天开始降解,C2组分从第36天开始降解,C3组分从第12天开始降解,C4组分荧光强度则不断在减少,C5组分荧光强度从初始阶段到第42天基本不变,从第42天以后呈现显著增加后保持稳定的趋势. C1~C4组分在20 W和40 W光照强度下的降解趋势也基本一致,但是降解速率有一定的差异.在20 W和40 W紫外照射下:C1组分光化学降解率分别为31.97 %、41.9 %,降解动力学常数分别为0.0072、0.013 d-1(R2>0.8);C2组分光化学降解率分别为32.17 %、47.49 %,降解动力学常数分别为0.0217、0.0344 d-1(R2>0.9);C3组分光化学降解率分别为33.47 %、64.94 %,降解动力学常数分别为0.0103、0.0214 d-1(R2>0.9);C4组分光化学降解率分别为40.69 %、80.36 %,降解动力学常数分别为0.012、0.0332 d-1(R2>0.9). 20 W和40 W光照强度下,4个组分的降解程度依次为:C4>C3>C2>C1,4个组分的降解速率均为C2>C4>C3>C1,表明光照强度对4个组分的光反应活性程度顺序并没有显著影响.
|
图 4 不同光照强度下各组分荧光强度随时间变化趋势 Fig.4 Variations in intensity of fluorescence components under different UV irradiation intensities |
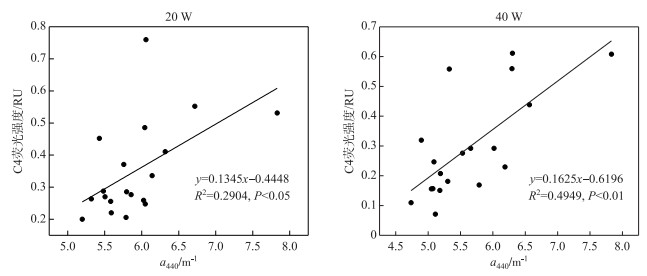
对5个组分的荧光强度和吸收系数a440做回归分析发现,C1、C2、C3、C5与a440不存在显著的相关关系,只有C4与a440呈显著的线性相关关系(图 5).
|
图 5 a440与C4组分荧光强度的相关性 Fig.5 The correlation between a440 and the fluorescent intensity of C4 |
表 2显示5个组分荧光强度之间的相关性分析,以40 W组为例.其中,C1与C2同为类腐殖质组分并在P < 0.01水平上显著正相关,表明C1与C2两组分的降解规律可能具有一定的相似性;C5与C2在P < 0.01水平上显著负相关,表明C5组分极有可能来源于C2组分的光降解.
| 表 2 各组分之间的相关关系 Tab. 2 The correlations among the different components |
随着光照时间的持续,腐殖酸溶液的a440、E4/E6呈下降趋势,说明腐殖酸在紫外光照射下发生了光降解,溶液浓度降低,同时生成了小分子物质.张运林等[32]在模拟UV-B辐射和自然光辐射CDOM实验过程中,发现CDOM的吸收系数a280随着UV-B光照时间增加而逐渐减少,SR随光照时间增加而增加.段洪涛等[33]利用440 nm处吸收系数a440研究太湖沿岸水体CDOM吸收光谱特性,发现a440与光谱斜率S呈现负相关性,与本研究结果一致. Santos等[34]的研究结果也表明Ria de Aveiro河口的CDOM的a254和a350随光照时间的增加而减小,而SR、S(275-295)呈增加趋势.本文所选取的a440、E4/E6和SR在P < 0.01水平上显著相关,都可被用来表征CDOM光降解过程中浓度和产物的变化情况.
腐殖酸不具有特定的结构和化学构型[35],多以脂肪碳、芳香碳为骨架,含有大量含氧官能团,如羟基、羟基、羰基、甲氧基、酚羟基、醛和酮等[36-38].光照能改变腐殖酸的分子结构,使得部分碳骨架和官能团发生破坏,与此同时物质的荧光性质也会发生改变.在对腐殖酸的荧光数据分析中,我们发现外源腐殖酸的初始荧光贡献中类腐殖质组分(C1、C2、C4)占71.88 %,类蛋白质组分(C3、C5)仅占28.12 %.以往的研究结果表明,外源有机质丰富的河流中类腐殖质荧光强度要大于类蛋白荧光强度[39-40],例如太湖、巢湖河口区的类腐殖质荧光强度峰值明显强于敞水区[40-41].实验结果显示UV类腐殖质、UVA类腐殖质、类色氨酸、UVC类腐殖质发生了紫外光降解,以往的实验结果多表明类腐殖质组分会发生明显的光降解现象[14, 17, 42],而类蛋白质组分对光照的响应则具有一定的争议. Zhang等[14]的实验结果表明类蛋白质组分比较难以发生光降解甚至出现浓度增加的现象,Berto等[43]发现光照后类络氨酸组分浓度出现增加的现象.一些学者认为类腐殖质组分可经光化学反应生成其他荧光组分[13-14, 30, 44],本实验中增加的类蛋白质组分浓度则也有可能来源于体系内其他组分的降解产物.而杨超等[45]则认为类蛋白质组分荧光浓度的升高来源于后含氮杂环被破坏后,肽链展开导致更多的荧光基团暴露于水中.光照强度对腐殖酸光降解行为有明显的影响,光照强度越大,其降解程度就越明显. 40 W组的a440、E4/E6的下降速率均高于20 W组;40 W组荧光组分(C1、C2、C3、C4)的光降解程度同样大于20 W组.随着光照强度的增大,光照能提供的光子增多,腐殖酸分子能吸收的光子相应增多,更多的腐殖酸分子被激发而被降解[46-47].
以往的水体溶解性有机碳的光照实验研究周期多集中于10天以内,短则只有几小时,本实验的研究周期长达54天,实验结果显示各个荧光组分的降解行为差异较大,造成这种现象的原因众多.首先,腐殖酸的光降解行为主要由其物质结构决定,例如,含有较多对UV有强吸收的芳香官能团的物质更容易被光降解[14],物质的供电子基团越多如羟基、酚基,其荧光猝灭的幅度就越大[48-49].而光降解过程中存在的光竞争行为[13]以及上述提到的荧光组分之间存在相互转化关系则也有可能是荧光组分降解速率和程度的差异的主要原因.根据本次实验结果,C2组分为类色氨酸,结构较为简单,容易发生光降解,因而其光降解速率很快;但是在光照过程中,不断有其他组分光降解后生成类色氨酸,导致其荧光强度先升高后下降,故C2组分的降解程度反而低于C4组分.另外,腐殖酸荧光特性的影响因子众多,包括盐度、温度、pH、溶解氧、流域水文情况等等[50-52],有研究结果表明荧光组分在得失电子的过程中也会出现荧光增强或猝灭的现象[45],因此体系中电子在5个组分之间不停地转移以及腐殖酸溶液体系的pH、溶解氧等参数的变化都会造成荧光信号的波动.本研究实验温度控制在25℃,接近于湖泊夏季温度.根据阿仑尼乌斯方程,可以预计在一定温度范围内随着水温的升高,反应活化分子数增多,有效碰撞增加,腐殖酸的降解速率加快[53].另外,腐殖酸溶液pH约为8,接近于湖泊天然pH.腐殖酸通常溶解于碱性溶液中,其溶解度随着pH升高而升高.假设在一定温度下,腐殖酸溶液初始浓度升高,反应物与反应物之间分子发生碰撞的几率较高,反应速率加快.但是腐殖酸光降解过程中的影响因子较多,各种实验参数的影响有待进一步验证.
光照可以显著改变湖泊生态系统中的物理、化学、生物过程.通常,伴随着有机物光降解过程水体的颜色将消失(即通常所称的光漂白作用),水色的改变影响水的光学特性和太阳辐射尤其紫外辐射在水中的穿透力,从而改变了水体生态系统的光学环境,影响水生生物的生命活动[54].此外,光照可以显著增加CDOM的生物利用性[10-12],进而被浮游细菌吸收利用,通过浮游动物或原生动物的牧食作用逐步向高营养级传递,促进湖泊的碳循环和物质流动.而湖泊生态系统作为自然界重要的碳库,光照强度的变化对于湖泊的碳循环过程可能产生重大的影响.研究光照强度变化对外源DOM降解行为的影响对于臭氧层损耗所导致的UVB辐射增强、日光辐射的季节变化对湖泊生态系统的影响等问题具有重要意义.因此后续应加强原位的、长时间尺度的外源DOM光降解及其对湖泊食物网碳循环影响方面的研究.
4 结论1) 通过分析腐植酸溶液光谱吸收系数a440、E4/E6值和光谱斜率比值SR可知,光降解过程中腐殖酸的结构发生了改变,产生了小分子物质. 40 W组的a440、E4/E6的下降速率均高于20 W组,表明光降解程度随着光照强度的增加而增加.
2) EEM-PARAFAC模型中共解析出5个组分,分别为UV类腐殖质、UVA类腐殖质、类色氨酸、UVC类腐殖质和类络氨酸.外源腐殖酸的初始荧光贡献中类腐殖质组分占71.88 %,类蛋白质组分占28.12 %.
3) 除了类络氨酸组分,其余4种组分均发生了光降解,4个组分光降解行为有较大差异,但光降解速率与程度均随着光照强度的增加而增加.随着光照强度的增大,光照能提供的光子增多,腐殖酸分子能吸收的光子相应增多,更多的腐殖酸分子被激发继而发生光裂解而被降解.
| [1] |
Fisher SG, Likens GE. Energy flow in bear brook, New Hampshire-integrative approach to stream ecosystem metabolism. Ecological Monographs, 1973, 43(4): 421-439. DOI:10.2307/1942301 |
| [2] |
Simon M, Cho BC, Azam F. Significance of bacterial biomass in lakes and the ocean comparison to phytoplankton biomass and biogeochemical implications. Marine Ecology Progress Series, 1992, 86(2): 103-110. DOI:10.3354/meps086103 |
| [3] |
Carpenter SR, Cole JJ, Pace ML et al. Ecosystem subsidies: terrestrial support of aquatic food webs from 13C addition to contrasting lakes. Ecology, 2005, 86(10): 2737-2750. DOI:10.1890/04-1282 |
| [4] |
Cole JJ, Karl DM. Strong evidence for terrestrial support of zooplankton in small lakes based on stable isotopes of carbon, nitrogen, and hydrogen. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(5): 1975. DOI:10.1073/pnas.1012807108 |
| [5] |
Arimitsu ML, Hobson KA, Webber DN et al. Tracing biogeochemical subsidies from glacier runoff into Alaska's coastal marine food webs. Global Change Biology, 2017, 24(1). DOI:10.1111/gcb.13875 |
| [6] |
Fellman JB, Hood E, Raymond PA et al. Evidence for the assimilation of ancient glacier organic carbon in a proglacial stream food web. Limnology and Oceanography, 2015, 60(4): 1118-1128. DOI:10.1002/lno.10088 |
| [7] |
Skoog A, Wedborg M, Fogelqvist E. Decoupling of total organic carbon concentrations and humic substance fluorescence in an extended temperate estuary. Marine Chemistry, 2011, 124(1/4): 68-77. DOI:10.1016/j.marchem.2010.12.003 |
| [8] |
Wetzel RG, Hatcher PG, Bianchi TS. Natural photolysis by ultraviolet irradiance of recalcitrant dissolved organic matter to simple substrates for rapid bacterial metabolism. Limnology and Oceanography, 1995, 40(8): 1369-1380. DOI:10.4319/lo.1995.40.8.1369 |
| [9] |
Lou T, Xie H. Photochemical alteration of the molecular weight of dissolved organic matter. Chemosphere, 2006, 65(11): 2333-2342. DOI:10.1016/j.chemosphere.2006.05.001 |
| [10] |
De Lange HJ, Morris DP, Williamson CE. Solar ultraviolet photodegradation of DOC may stimulate freshwater food webs. Journal of Plankton Research, 2003, 25(1): 111-117. DOI:10.1093/plankt/25.1.111 |
| [11] |
Amado AM, Farjalla VF, Esteves FDA et al. Complementary pathways of dissolved organic carbon removal pathways in clear-water Amazonian ecosystems: photochemical degradation and bacterial uptake. FEMS Microbiology Ecology, 2010, 56(1): 8-17. DOI:10.1111/j.1574-6941.2006.00028x |
| [12] |
Su Y, Hu E, Feng M et al. Comparison of bacterial growth in response to photodegraded terrestrial chromophoric dissolved organic matter in two lakes. Science of the Total Environment, 2016, 579: 1203-1214. DOI:10.1016/j.scitotenv.2016.11.104 |
| [13] |
Du Y, Zhang Y, Chen F et al. Photochemical reactivities of dissolved organic matter (DOM) in a sub-alpine lake revealed by EEM-PARAFAC: An insight into the fate of allochthonous DOM in alpine lakes affected by climate change. Science of the Total Environment, 2016, 568: 216-225. DOI:10.1016/j.scitotenv.2016.06.036 |
| [14] |
Zhang Y, Liu X, Osburn CL et al. Photobleaching response of different sources of chromophoric dissolved organic matter exposed to natural solar radiation using absorption and excitation-emission matrix spectra. PLoS One, 2013, 8(10): e77515. DOI:10.1371/journal.pone.0077515 |
| [15] |
Hu SZ, Li GJ, Li YJ et al. Optical absorption properties of chromophoric dissolved organic matter and the tracing implication of dissolved organic carbon in the Yangtze River estuary. Journal of Tianjin University of Science & Technology, 2015, 30(3): 57-61. [胡素征, 李桂菊, 李奕洁等. 长江口有色溶解有机物光谱特性及其示踪溶解有机碳研究. 天津科技大学学报, 2015, 30(3): 57-61.] |
| [16] |
Huang CC, Li YM, Wang Q et al. Components optical property of CDOM in Lake Taihu based on three-dimensional excitation matrix fluorescence. J Lake Sci, 2010, 22(3): 375-382. [黄昌春, 李云梅, 王桥等. 基于三维荧光和平行因子分析法的太湖水体CDOM组分光学特征. 湖泊科学, 2010, 22(3): 375-382. DOI:10.18307/2010.0309] |
| [17] |
Yan LH, Su RG, Shi XY et al. Assessing the dynamics of chromophoric dissolved organic matter (CDOM) in the Yellow Sea and the East China Sea in autumn by EEMs-PARAFAC. Science China Chemistry, 2012, 55(12): 2595-2609. DOI:10.1007/s11426-012-4617-7 |
| [18] |
Stedmon CA, Markager S, Bro R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Marine Chemistry, 2003, 82(3/4): 239-254. DOI:10.1016/S0304-4203(03)00072-0 |
| [19] |
Li P, Chen L, Zhang W et al. Spatiotemporal distribution, sources, and photobleaching imprint of dissolved organic matter in the Yangtze Estuary and its adjacent sea using fluorescence and parallel factor analysis. PLoS One, 2015, 10(6): e0130852. DOI:10.1371/journal.pone.0130852 |
| [20] |
Solomon CT, Jones SE, Weidel BC et al. Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: current knowledge and future challenges. Ecosystems, 2015, 18(3): 376-389. DOI:10.1007/s10021-015-9848-y |
| [21] |
Mcdonald S, Bishop AG, Prenzler PD et al. Analytical chemistry of freshwater humic substances. Analytica Chimica Acta, 2004, 527(2): 105-124. DOI:10.1016/j.aca.2004.10.011 |
| [22] |
Stedmon CA, Markager S, Kaas H. Optical properties and signatures of chromophoric dissolved organic matter (CDOM) in Danish coastal waters. Estuarine Coastal and Shelf Science, 2000, 51(2): 267-278. DOI:10.1006/ecss.2000.0645 |
| [23] |
Polak J, Bartoszek M, Sulkowski WW. Comparison of humification processes occurring during sewage purification in treatment plants with different technological processes. Water Research, 2009, 43(17): 4167-4176. DOI:10.1016/j.watres.2009.06.040 |
| [24] |
Helms JR, Stubbins A, Ritchie JD et al. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnology and Oceanography, 2008, 53(3): 955-969. DOI:10.2307/40058211 |
| [25] |
Guo WD, Cheng YY. Photodegradation of chromophoric dissolved organic matter from Jiulong River Estuary under natural solar radiation. Environmental Science, 2008, 29(6): 1463-1468. [郭卫东, 程远月. 天然日光辐照下河口区CDOM的光化学降解. 环境科学, 2008, 29(6): 1463-1468. DOI:10.13227/j.hjkx.2008.06.007] |
| [26] |
Cory RM, Mcknight DM. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environmental Science & Technology, 2005, 39(21): 8142-8149. DOI:10.1021/es0506962 |
| [27] |
Murphy KR, Ruiz GM, Dunsmuir WT et al. Optimized parameters for fluorescence-based verification of ballast water exchange by ships. Environmental Science and Technology, 2006, 40(7): 2357-2362. DOI:10.1021/es0519381 |
| [28] |
Stedmon CA, Markager S. Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using parafac analysis. Limnology and Oceanography, 2005, 50(2): 686-697. DOI:10.4319/lo.2005.50.2.0686 |
| [29] |
Cheng YY, Guo WD. Characterization of Photobleaching of chromophoric dissolved organic matter in Xiamen bay by excitation emission matrix spectroscopy. Spectroscopy and Spectral Analysis, 2009, 29(4): 990-993. [程远月, 郭卫东. 厦门湾有色溶解有机物光漂白的三维荧光光谱研究. 光谱学与光谱分析, 2009, 29(4): 990-993. DOI:10.3964/j.issn.1000-0593(2009)04-0990-04] |
| [30] |
Coble PG. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Marine Chemistry, 1996, 51(4): 325-346. DOI:10.1016/0304-4203(95)00062-3 |
| [31] |
Van Housed MC, Thompson F, Dennis J et al. Distribution and optical properties of CDOM in the Arabian Sea during the 1995 Southwest Monsoon. Deep Sea Research Part Ⅱ Topical Studies in Oceanography, 1998, 45(10/11): 2195-2223. DOI:10.1016/S0967-0645(98)00068-X |
| [32] |
Zhang YL, Liu ML, Qin BQ et al. Photochemical degradation of chromophoric-dissolved organic matter exposed to simulated UV-B and natural solar radiation. Hydrobiologia, 2009, 627(1): 159-168. DOI:10.1007/s10750-009-9722-z |
| [33] |
Duan HT, Ma RH, Kong WJ et al. Optical properties of chromophoric dissolved organic matter in Lake Taihu. J Lake Sci, 2009, 21(2): 242-247. [段洪涛, 马荣华, 孔维娟等. 太湖沿岸水体CDOM吸收光谱特性. 湖泊科学, 2009, 21(2): 242-247. DOI:10.18307/2009.0213] |
| [34] |
Santos L, Santos EB, Dias JM et al. Photochemical and microbial alterations of DOM spectroscopic properties in the estuarine system Ria De Aveiro. Photochemical & Photobiological Sciences, 2014, 13(8): 1146-1159. DOI:10.1039/c4pp00005f |
| [35] |
Yan SX, Liu CH, Liang Y. Review on structural properties and multiple functionalities of humic acids. Natural Product Research and Development, 2017, 29(3): 511-516. [闫淑霞, 刘春花, 梁岩. 腐殖酸的结构特性与应用研究进展. 天然产物研究与开发, 2017, 29(3): 511-516.] |
| [36] |
Wang W, Li H, Ding Z et al. Effects of advanced oxidation pretreatment on residual aluminum control in high humic acid water purification. Journal of Environmental Sciences, 2011, 23(7): 1079-1085. DOI:10.1016/S1001-0742(10)60520-7 |
| [37] |
Liu CC, Han Y, Zhuo Y et al. Chemical composition and structural characteristics of humic acid extracted from excess sludge in municipal wastewater treatment plants. Environmental Chemistry, 2017, 36(8): 1735-1743. [刘超超, 韩芸, 卓杨等. 城市污水厂剩余污泥中腐殖酸化学组成与结构特征. 环境化学, 2017, 36(8): 1735-1743.] |
| [38] |
Song HY, Yin YY, Song JZ. The chemical composition and structure of humic acid from different enviroments. Journal of South China Normal University: Nature Science Edition, 2009(1): 61-66. [宋海燕, 尹友谊, 宋建中. 不同来源腐殖酸的化学组成与结构研究. 华南师范大学学报:自然科学版, 2009(1): 61-66.] |
| [39] |
Baker A. Fluorescence excitation-emission matrix characterization of river waters impacted by a tissue mill effluent. Environmental Science & Technology, 2002, 36(7): 1377-1382. DOI:10.1021/es0101328 |
| [40] |
Liu ML, Zhang YL, Qin BQ. Characterization of absorption and three-dimensional excitation-emission matrix spectra of chromophoric dissolved organic matter at the river inflow and the open area in Lake Taihu. J Lake Sci, 2009, 21(2): 234-241. [刘明亮, 张运林, 秦伯强. 太湖入湖河口和开敞区CDOM吸收和三维荧光特征. 湖泊科学, 2009, 21(2): 234-241. DOI:10.18307/2009.0212] |
| [41] |
Wang ZG, Liu WQ, Li HB et al. Analysis of CDOM spatial distributions variations in Chaohu Lake and its sources by three-dimensional fluorescence excitation-emission matrix. Acta Scientiae Circumstantiae, 2006, 26(2): 275-279. [王志刚, 刘文清, 李宏斌等. 三维荧光光谱法分析巢湖CDOM的空间分布及其来源. 环境科学学报, 2006, 26(2): 275-279. DOI:10.3321/j.issn:0253-2468.2006.02.019] |
| [42] |
Hernes PJ, Benner R. Photochemical and microbial degradation of dissolved lignin phenols: implications for the fate of terrigenous dissolved organic matter in marine environments. Journal of Geophysical Research Oceans, 2003, 108(C9). DOI:10.1029/2002JC001421 |
| [43] |
Berto S, Isaia M, Sur B et al. UV-vis spectral modifications of water samples under irradiation: Lake vs. subterranean water. Journal of Photochemistry & Photobiology A Chemistry, 2013, 251(9): 85-93. DOI:10.1016/j.jphotochem.2012.10.019 |
| [44] |
Helms JR, Stubbins A, Perdue EM et al. Photochemical bleaching of oceanic dissolved organic matter and its effect on absorption spectral slope and fluorescence. Marine Chemistry, 2013, 155(4): 81-91. DOI:10.1016/j.marchem.2013.05.015 |
| [45] |
Yang C, He XS, Xi BD et al. Effect of electron transfer on the structure of dissolved organic matter during initial landfill stage. China Enviromental Science, 2017, 37(1): 229-237. [杨超, 何小松, 席北斗等. 填埋初期水溶性有机物结构受电子转移的影响. 中国环境科学, 2017, 37(1): 229-237.] |
| [46] |
Kieber RJ, Zhou X, Mopper K. Formation of carbonyl compounds from UV-induced photodegradation of humic substances in natural waters: Fate of riverine carbon in the sea. Limnology and Oceanography, 1990, 35(7): 1503-1515. DOI:10.4319/lo.1990.35.7.1503 |
| [47] |
Yang DT, Chen WM, Wu SC et al. The effects of CDOM on the attenuation of close ultraviolet and blue light in Chaohu Lake and Longgan Lake. J Lake Sci, 2003, 15(3): 269-274. [杨顶田, 陈伟民, 吴生才等. 湖泊中有色可溶性物质对近紫外及蓝光衰减的影响. 湖泊科学, 2003, 15(3): 269-274. DOI:10.18307/2003.0312] |
| [48] |
Ma J, Del VR, Golanoski KS et al. Optical properties of humic substances and cdom: Effects of borohydride reduction. Environmental Science & Technology, 2010, 44(14): 5395-5402. |
| [49] |
Boyle ES, Nicolas G, Anthony T et al. Optical properties of humic substances and cdom: Relation to structure. Environmental Science & Technology, 2009, 43(7): 2262-2268. |
| [50] |
Liu XH, Zhang YL, Yin Y et al. Application of three-dimensional fluorescence spectroscopy and parallel factor analysis in CDOM study. Transactions of Oceanology and Limnology, 2012(3): 133-145. [刘笑菡, 张运林, 殷燕等. 三维荧光光谱及平行因子分析法在CDOM研究中的应用. 海洋湖沼通报, 2012(3): 133-145.] |
| [51] |
Qi L, Xie H, Gagné JP et al. Photoreactivities of two distinct dissolved organic matter pools in groundwater of a subarctic island. Marine Chemistry, 2018, 202: 97-120. DOI:10.1016/j.marchem.2018.03.003 |
| [52] |
Song G, Li Y, Hu S et al. Photobleaching of Chromophoric Dissolved Organic Matter (CDOM) in the Yangtze River estuary: Kinetics and effects of temperature, pH, and salinity. Environmental Science Processes & Impacts, 2017, 19(6): 861-873. |
| [53] |
Sierra CA. Temperature sensitivity of organic matter decomposition in the Arrhenius equation: some theoretical considerations. Biogeochemistry, 2012, 108(1/2/3): 1-15. DOI:10.1007/s10533-011-9596-9 |
| [54] |
Zhang YL. Advances in chromophoric dissolved organic matter in aquatic ecosystems. Transactions of Oceanology and Limnology, 2006(3): 119-127. [张运林. 水体中有色可溶性有机物的研究进展. 海洋湖沼通报, 2006(3): 119-127. DOI:10.3969/j.issn.1003-6482.2006.03.018] |
 2019, Vol. 31
2019, Vol. 31 

