(2: 暨南大学生态系, 广州 510632)
(3: 中国-丹麦科研教育中心, 北京 100190)
(4: 华南师范大学地理科学学院, 广州 510631)
(2: Department of Ecology, Jinan University, Guangzhou 510632, P. R. China)
(3: Sino-Danish Center for Education and Research, Beijing 100190, P. R. China)
(4: South China Normal University, Guangzhou 510631, P. R. China)
浅水湖泊中初级生产者主要有藻类、水生维管束植物等,主要分布于底栖和敞水两种类型的生境中.分布于底栖生境中的植物主要包括维管束挺水植物、沉水植物、浮叶植物以及底栖大型藻类和微藻等,因挺水植物和浮叶植物往往分布范围有限,沉水植物和底栖藻类往往是优势类群[1].生活在敞水生境中的植物主要为藻类,即浮游植物.光和营养盐是影响湖泊植物生长的主要因素,底栖植物和浮游植物之间存着竞争关系,在不同的湖泊环境条件下的竞争优势不同.相较于沉积物,湖水中营养盐含量较低.浮游植物悬浮在湖水中,常常受到营养盐的限制,而在光的竞争方面有优势.由于底层光照受到湖水和水中各种物质的影响,底栖植物生长容易受到光的限制,但是因其可以从沉积物中直接获取营养盐,在营养盐获取方面有明显的优势[2-5].贫营养浅水湖泊中沉水植物和底栖藻类往往是湖泊的优势初级生产者.随着富营养化的加剧,浮游植物可以逐步从湖水中获得更多的营养盐,生物量逐步升高,水体浊度增加,透明度下降,其对底栖植物的遮蔽作用逐步增强[6].也就是说,随着富营养化的加剧,浮游植物的竞争优势越来越强,而沉水植物和底栖藻类的竞争优势越来越弱,最终导致浅水湖泊生态系统从以底栖植物为优势的清水态转换为以浮游植物为优势的浑水态,即所谓的稳态转换[7].所以,稳态转换实际是底栖初级生产者和浮游初级生产者优势度的转换[8].
浅水湖泊稳态转换过程中,生物、化学和物理特征及过程不断发生变化,其协同作用形成了促进这种稳态转换的驱动力,使原有的底栖-敞水生境耦合发生变化,包括营养盐交换过程等,逐步向有利于浮游植物优势的方向发展[4, 7].由于磷是湖泊生态系统的限制因子,磷循环是沉积型循环,一般没有气体状态[9-10],因此底栖初级生产者与浮游生产者优势转换主要由沉积物和湖水之间磷交换过程的特征所决定.在进行湖泊修复时,必须了解这些关键过程,针对这些过程采取措施才能取得良好的效果.
本文以磷循环和初级生产者变化为主线,综述富营养化对浅水湖泊底栖-敞水生境耦合过程的影响,并探讨稳态转换的机理及其对湖泊修复技术研究与工程应用的启示.
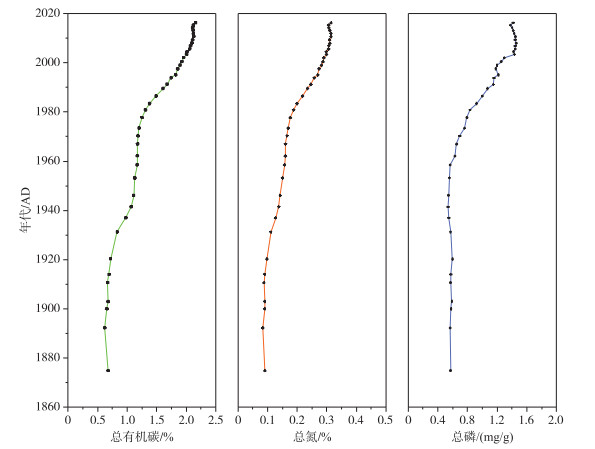
1 富营养化过程中浅水湖泊关键理化与生物特征变化及其对底栖-敞水生境耦合的影响 1.1 沉积物理化特征的变化及其影响在自然(营养水平低的)湖泊中,磷进入湖泊后大部分会通过各种途径进入沉积物[11].颗粒磷通过沉降进入沉积物,溶解态磷可被其他无机和有机颗粒物吸附或被生物利用变成颗粒磷后沉降到沉积物,因此沉积物常是磷循环的汇[10].随着人类活动的加剧,进入湖泊的外源磷负荷增加,沉积物中的磷含量明显提高[12].如位于长江中游的太白湖,沉积物氮、磷等营养盐含量逐年升高,尤其是改革开放后呈现出快速上升的趋势(图 1).由于初级生产力的提高,沉积物有机质含量和含水率也随之提高[13-14].
|
图 1 长江中游太白湖沉积物中总有机碳、总氮和总磷含量的历史变化[14] Fig.1 Historical changes of total organic carbon, total nitrogen and total phosphorus contents in the sediment of Lake Taibai in the middle reaches of the Yangtze River |
沉积物中的磷可以通过扩散(溶解态磷)和再悬浮(颗粒态磷)等途径进入湖水(敞水生境),其通量与沉积物磷含量往往呈显著正相关关系[11].同时由于沉积物含水率的提高,密度下降,在同样的水动力条件下,沉积物再悬浮的速率较高,随再悬浮进入湖水中的磷通量也会增加[15].此外,沉积物有机质的增加会促进微生物的活动、加速磷的矿化过程,并降低沉积物表层的氧化还原条件,增加沉积物中溶解态磷的含量,从而提高沉积物磷的释放速率,这是湖泊磷内源负荷形成的重要过程[15-16].沉积物磷进入湖水中的通量(内源负荷)增加无疑会促进浮游植物的生长,而沉积物再悬浮同时还会提高水体的浊度,削弱底栖植物的竞争优势,而有利于提高浮游植物的优势度.
1.2 浮游植物的变化及其影响浮游植物悬浮在敞水生境中,从湖水中获取营养盐.磷等营养盐外源负荷的增加会提高湖水中营养盐的浓度,从而促进浮游植物的生长,因此,浮游植物生物量与总磷浓度往往有较好的正相关关系[17-18].在贫营养湖泊中,金藻往往是浮游植物群落的优势类群,随富营养化程度的增加,其优势逐步减小,而羽纹纲硅藻的优势度逐步上升.随着富营养化进一步发展,蓝藻的数量增加,甚至暴发水华[19].而Jensen等[20]发现,在超富营养浅水湖泊中绿藻往往占优势,而蓝藻的优势度会下降.
浮游植物生物量的增加,首先会增加湖水的浊度,加速湖水中光的衰减,从而抑制沉水植物和底栖藻类的生长,降低底栖初级生产力.浮游植物生长时吸收湖水中的营养盐,而碎屑一部分会沉降到沉积物中,这是湖水中营养盐进入沉积物的主要途径之一[21].另外,由于有机物的增加会改变沉积物氧化还原条件等理化性质,促进沉积物营养盐的释放[22].再者,浮游植物的光合作用会提高湖水的pH等,接触到沉积物表层,也会促进沉积物磷的释放[15, 23].这些变化均有利于沉积物中磷向湖水中输移,形成有利于浮游植物生长的正反馈作用.
1.3 沉水植物与底栖藻类的变化及其影响沉水植物和底栖藻类由于能从沉积物中直接获取营养盐[24],常在水质清澈的贫营养浅水湖泊中占优势.随着外源营养盐输入的增加,湖水中营养盐的可得性增加,从而有利于浮游植物的生长.随着浮游植物生物量的增加,对沉水植物和底栖藻类的遮蔽作用增强,使其生长受到的光抑制逐步加强,竞争优势不断减弱[25-26].浮游植物生活在水中,能获得较好的光照条件,一些种类甚至可以通过调节自身的浮力或通过垂直运动,在光照较好和营养盐较高的水层之间进行垂直迁移,从而提高其竞争优势[27].由于沉水植物也能从水中吸收营养盐,与浮游植物存在营养盐竞争,沉水植物减少可使浮游植物从水中获得更多营养盐[3].然而,浅水湖泊中沉水植物和底栖藻类的减少或消失导致沉积物磷等营养盐的释放增加,能给浮游植物生长提供更多的营养盐,这也是稳态转换后湖水中磷浓度和浮游植物生物量大幅增加的主要原因[26].
Vadeboncoeur等[8]发现,在格陵兰浅水湖泊中,底栖(包括附着)藻类的生产力占全湖初级生产力的80 % ~98 %,而在总磷较高的丹麦浅水湖泊中浮游植物生产力占全湖初级生产力的比例接近100 % (图 2).

|
图 2 格陵兰、美国和丹麦不同湖泊中底栖藻类初级生产力占全湖初级生产力的百分比随总磷浓度的上升而下降[8] Fig.2 The relative contribution of benthic algae to whole-lake primary production as a function of total phosphorus concentrations in lakes from Greenland, USA and Denmark |
因此,富营养化往往会驱动湖泊从底栖植物为主的生态系统向浮游植物为主的生态系统转变,并影响底栖-浮游生境间磷的交换:
1) 沉水植物消失对水动力条件和沉积物再悬浮的影响.研究表明,沉水植物的消失会提高水的流速和紊流强度[24, 28],促进沉积物再悬浮和物质输移扩散[25, 29-30].沉水植物还可以促进悬浮物(包括浮游植物)的沉降,减少水中营养盐含量,其消失会降低悬浮物的沉降速率[3, 31].
2) 沉水植物和底栖藻类减少和消失可以改变表层沉积物的性质,促进沉积物磷释放.沉水植物和底栖藻类都可以提高沉积物表层氧化还原电位,从而抑制铁磷的释放[32]. Zhang等[33]发现,有沉水植物的湖泊沉积物磷释放速率显著低于无沉水植物的湖泊沉积物,而底栖藻类有同样的作用.因此,沉水植物和底栖藻类的消失会大幅提高湖泊内源营养盐负荷,为浮游植物的发展提供更多营养盐.由此可见,浅水湖泊浮游植物不仅因为底栖植物下降减少了竞争,从湖水中获得更多营养盐,还因底栖植物对沉积物磷释放的抑制作用减弱或消失,导致内源磷负荷的增加,从而获得更多的营养盐补充.
因此,沉水植物和底栖藻类是维持清水态,从而维持敞水生境(水柱)较低磷浓度和较低浮游植物生物量的关键因子,也是影响沉积物磷滞留能力的关键因子之一.富营养化逐步减弱底栖植物在浅水湖泊中的优势,沉积物对磷的滞留能力下降,同时水柱中磷的沉降速率也下降.因此,富营养化导致底栖生境中的磷向敞水生境中转移的净通量(内源负荷)增加,改变了清水态湖泊中底栖-敞水生境之间磷交换的平衡.
1.4 底栖动物的变化及其影响随着富营养化的加剧,浅水湖泊大型底栖动物群落常常发生显著的变化.螺类、双壳类等由于基质(大型植物)较少或生境质量恶化(缺氧程度高)等因素,数量会减少;而寡毛类等密度会上升[34-36].研究表明,双壳类可以降低敞水生境中的悬浮物浓度,提高透明度,从而有利于底栖植物的生长以及湖泊清水态的维持[37-38].而寡毛类等可能通过增加沉积物磷释放[39]、影响底栖藻类生长等途径提高浮游植物生产力[38].一些螺类不仅可以控制沉水植物表面的附着藻类,促进沉水植物的生长,也可以降低浮游植物的密度[40-41].因此,大型底栖动物群落中双壳类和螺类的减少、寡毛类增多会促进浮游初级生产力发展,而抑制底栖初级生产力,从而促进湖泊从清水态向浑水态的转变[38].
底栖食物网结构无疑对浅水湖泊生态系统有重要影响,而现有关于浅水食物网的研究主要集中在敞水食物网.相关底栖食物网的研究主要关注螺类和双壳类对附着藻类、浮游植物和沉水植物等初级生产者的影响[41-43].少数研究关注到包括鱼类等更高营养级的消费者,但有关底栖食物网结构变化对整个生态系统的影响报道较少[44-45].
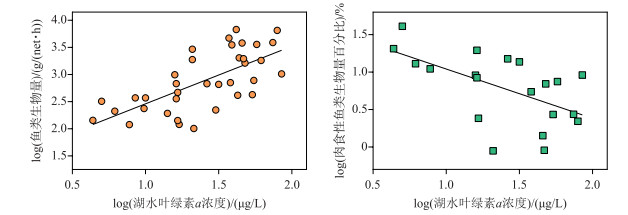
1.5 鱼类的变化及其影响随着富营养化的加剧,鱼类总生物量增加,同时鱼类群落结构也会发生变化. Jeppesen等[46]发现,丹麦温带浅水湖泊中浮游生物食性鱼类生物量(单位努力渔获量)随湖泊总磷浓度的升高而增加,而肉食性鱼类的比例下降(图 3).我国亚热带东部浅水湖泊的调查也发现,鱼类生物量随浮游植物生物量(叶绿素a浓度)的升高而增加,而肉食性鱼类生物量比例下降(图 4).

|
图 3 丹麦浅水湖泊浮游生物食性鱼类和肉食性鱼类生物量比例与总磷浓度的关系[46] Fig.3 The relationships of the biomass of planktivorous fish and the percentage of piscivorous fish with the total phosphorus concentrations in Danish shallow lakes |
|
图 4 我国东部浅水湖泊鱼类生物量(单位努力渔获量)和肉食性鱼类生物量百分比(%)与叶绿素a浓度的关系 Fig.4 The relationships of the fish biomass (catch per unit effort) and the percentage of piscivorous fish with the chlorophyll-a concentrations in shallow lakes of eastern China |
鱼类群落结构变化会影响浅水湖泊生态系统的结构与功能.鱼类最重要的影响之一是对水动力条件的影响,鱼类密度的增加会提高水的紊动强度,从而影响沉积物的再悬浮和营养盐等物质的扩散[47].底栖生物食性鱼类还可以通过摄食活动促进沉积物的再悬浮,如鲤摄食时会把沉积物与食物同时摄入口中,通过鳃耙滤取食物,而沉积物通过鳃耙后进入湖水中,从而导致沉积物再悬浮[48-50].研究同时表明,鱼类扰动也会影响沉积物表层和下层的混合深度,也使沉积物下层的活性磷释放风险增加[51]. Scheffer等[52]发现,鱼类扰动会降低沉积物的稳定性和抗侵蚀能力,从而促进沉积物再悬浮、降低水体透明度.
浮游生物食性鱼类同样对沉积物营养盐释放有促进作用. Zhang等[33]运用放射性同位素示踪技术研究了鲢对富营养化湖泊沉积物磷释放的影响,结果发现鲢会减少底栖藻类的生物量,提高沉积物磷释放速率,从而提高水中磷和浮游植物(叶绿素a)的浓度. Guo等[53]在太湖的围隔实验也发现,鲢、鳙虽然降低了蓝藻的比例,却提高了湖水的总磷浓度.
鱼类群落结构的变化还会对食物网结构产生重要影响.如上所述,肉食性鱼类减少会导致浮游动物食性鱼类的增多,大型浮游动物数量也随之减少,对浮游植物的下行控制作用减弱,间接影响底栖初级生产力[54-55].在我国热带亚热带地区浅水湖泊鱼类丰富,很多鱼类繁殖期长,幼鱼密度高,对大型浮游动物的捕食压力大,大型浮游动物(如溞属种类)难以建立较高的种群密度,食物网对浮游植物控制的下行效应十分有限或短暂[56-58].
浊度的提高、对沉积物表层扰动与营养盐释放增加等过程的变化不利于沉水植物和底栖藻类的生长[24, 59],而浮游植物能因此获得更多竞争优势,从而促进浅水湖泊生态系统从清水态向浑水态的转换,鱼类的这种影响随着富营养化的发展而加剧.
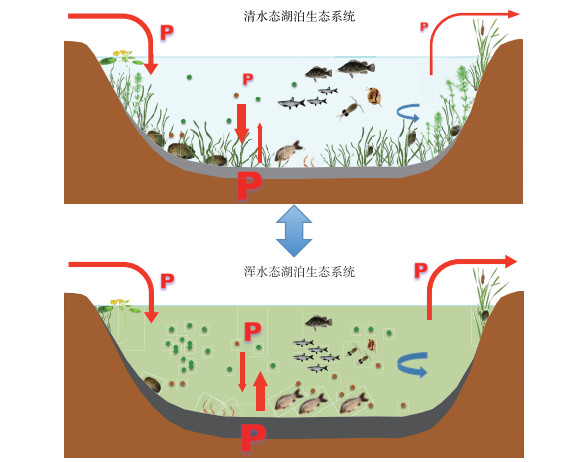
综上所述,外源营养盐负荷增加引起的富营养化驱动着浅水湖泊生态系统结构和功能的演变.湖水中营养盐的增多促进了浮游植物的生长,由于其遮蔽作用等导致底栖植物减少,而底栖植物的减少又导致沉积物中磷释放增加,浮游植物获得更多营养盐.同时底栖植物的减少和鱼类的增多也提高了沉积物再悬浮速率,进一步提高湖水浊度和营养盐浓度而不利于底栖植物的生长.底栖动物和鱼类群落也在富营养化的驱动下发生变化,通过影响沉积物磷释放、沉积物再悬浮和沉降等过程而削弱底栖植物的竞争优势.富营养化驱动的这一系列反馈机制或作用影响着底栖-敞水生境耦合作用,营养盐交换通量更多流向敞水生境,湖泊逐步从以底栖植物为优势转变为以浮游植物为优势的系统,最终实现稳态转换(图 5).
|
图 5 浅水湖泊生态系统稳态转换:富营养化驱动以底栖植物为优势的清水态(上图)转变为以浮游植物为优势的浑水态(下图),修复的目标则是重建清水态;底栖-敞水生境之间的磷交换特征是控制湖水磷浓度以及湖泊状态的关键(P表示磷,字母大小表示相对含量;红色箭头表示磷输移方向,粗细表示通量大小;蓝色箭头表示水动力条件,粗细表示流速大小和紊流强度;绿色圆点表示浮游植物;褐色圆点表示悬浮物) Fig.5 Regime shifts in shallow lake ecosystems: Eutrophication drives the clear water state dominated by benthic plants (above) to turbid water state dominated by phytoplankton (below), while the goal of restoration is to re-establish the clear water state; the processes of phosphorus exchange between benthic and pelagic habitats are the key to control the concentrations of phosphorus in lake water and the status of the lakes (P indicates phosphorus and the size indicate relative content; red arrows indicate the direction of phosphorus transport, the thickness indicates the relative flux; blue arrows indicate hydrodynamic condition, the thickness indicates velocity size and turbulence intensity; green dots indicate phytoplankton; brown dots indicate suspended solids) |
在外源营养盐负荷得到有效控制后底栖-敞水生境之间的营养盐交换特征是控制湖水磷浓度的关键,而生境间的交换特征与生态系统特征(结构)密切相关(图 5).由于上述一系列反馈机理的作用,浑水态系统表现出较好的稳定性,这也是控制外源营养盐负荷后浅水湖泊恢复效果不理想的主要原因.
2 浅水湖泊底栖-敞水生境耦合过程的变化对湖泊修复的意义 2.1 对确定浅水湖泊生态系统恢复目标的意义湖泊修复的关键是实现从浑水态向清水态的转变,即从浮游初级生产力为主转变到以底栖初级生产力为主.而且由于一系列的正反馈作用,底栖初级生产力的优势越明显,清水态系统就越稳定.在我国湖泊治理实践中,核心目标还是以营养盐等化学指标为主,而这些指标的实现能否重建湖泊的清水态其实是个未知数.因此,即使在一些外源污染已经得到较好控制的湖泊,清水态生态系统还是难以建立,营养盐等指标也难以得到显著改善.尤其是在夏季,由于磷内源负荷的影响,湖水磷浓度可能与治理前相比没有显著变化[57, 60].因此,在制定浅水湖泊恢复目标时,要考虑建立和维持以清水态为目标的指标体系,如透明度、沉水植物覆盖度、浮游植物生物量等.
2.2 对确定湖泊修复关键技术研究和工程应用的意义稳态转换,即底栖和敞水生境中初级生产者优势的转换,受底栖-敞水生境耦合过程的控制,而这种耦合过程是由湖泊理化性质和生物群落结构特征所决定的,如沉水植物消失导致的湖水中磷沉降和沉积物中磷释放的变化等.
要成功修复富营养浅水湖泊,实现向清水态的转变,在外源营养盐负荷得到有效控制后,湖泊理化特征和生物群落结构必须逐步得到恢复,从而恢复原有功能.而一些湖泊生态系统结构的自然恢复可能十分缓慢.如湖泊沉积物磷的释放可使湖泊长期维持在较高营养状态,要恢复到以前的磷水平可能需要数十年以上[61].鱼类群落结构的变化也十分缓慢,湖泊营养盐输入减少和初级生产力降低,鱼类由于食物减少,其繁殖和生长会逐步降低,而死亡率会上升,由于鱼类的生命周期长,同时鱼类对湖泊产生影响,形成对自身有利的正反馈作用(如促进沉积物营养盐释放,而提高湖泊生产力),一些鱼类种群的响应会十分缓慢,数年或数十年才能看到显著变化[46].因此,通过一些湖泊修复技术或措施(如人工去除底栖生物食性鱼类等),能促进湖泊的恢复,小型湖泊甚至能在数月内重建清水态生态系统[50, 58, 60].
湖泊修复的关键技术必须是针对控制湖泊底栖-敞水生境耦合关键过程的相关技术,并具有较高的可操作性.技术的实施可以大幅削弱维持浑水态的正反馈作用,而逐步恢复清水态,建立维持清水态的反馈作用.根据富营养化对底栖-敞水生境耦合作用的影响,浅水湖泊修复的关键技术研究与工程应用应该重点关注以下几个方面:1)沉积物磷释放与再悬浮的控制技术:目标是控制底栖生境中的营养盐向敞水生境输入,从而控制浮游初级生产力,促进系统向有利于底栖初级生产者形成优势的方向发展. 2)鱼类群落调控技术:尤其是控制对沉积物扰动较大的鱼类种群,减少沉积物再悬浮的同时,也有利于沉水植物和底栖藻类的生长. 3)沉水植物群落恢复技术:在人工协助下加速沉水植物群落的恢复,同时促进底栖藻类的发展,最终形成底栖初级生产者占主导的浅水湖泊生态系统,即清水态浅水湖泊生态系统.
| [1] |
Kalff J ed. Translated by Gu BH, Liu ZW, Li KY et al. Limnology: Inland water ecosystems. Beijing: Higher Education Press, 2011: 122-126. [Kalff J编.古滨河, 刘正文, 李宽意等译.湖沼学: 内陆水生态系统.北京: 高等教育出版社, 2011: 122-126. ]
|
| [2] |
Rattray MR, Howard-Williams C, Brown JMA. Sediment and water as sources of nitrogen and phosphorus for submerged rooted aquatic macrophytes. Aquatic Botany, 1991, 40(3): 225-237. DOI:10.1016/0304-3770(91)90060-i |
| [3] |
Yang QX. Studies on the interaction of submerged plant and phytoplankton in eutrophic waters. J Lake Sci, 1996, 8(S1): 17-24. [杨清心. 富营养水体中沉水植物与浮游藻类相互竞争的研究. 湖泊科学, 1996, 8(S1): 17-24. DOI:10.18307/1996.sup03] |
| [4] |
Vadeboncoeur Y, Lodge DM, Carpenter SR. Whole-lake fertilization effects on distribution of primary production between benthic and pelagic habitats. Ecology, 2001, 82(4): 1065-1077. DOI:10.1890/0012-9658(2001)082[1065:wlfeod]2.0.co;2 |
| [5] |
Flöder S, Combüchen A, Pasternak A et al. Competition between pelagic and benthic microalgae for phosphorus and light. Aquatic Sciences, 2006, 68(4): 425-433. DOI:10.1007/s00027-006-0824-7 |
| [6] |
Jäger CG, Diehl S. Resource competition across habitat boundaries: Asymmetric interactions between benthic and pelagic producers. Ecological Monographs, 2014, 84(2): 287-302. DOI:10.1890/13-0613.1 |
| [7] |
Scheffer M ed. Ecology of shallow lakes. Springer Science & Business Media, 1997.
|
| [8] |
Vadeboncoeur Y, Jeppesen E, Zanden MJV et al. From Greenland to green lakes: Cultural eutrophication and the loss of benthic pathways in lakes. Limnology and Oceanography, 2003, 48(4): 1408-1418. DOI:10.4319/lo.2003.48.4.1408 |
| [9] |
Wang XR, Ding LL, Niu XJ et al. Roles of phosphine in the biogeochemical cycling of phosphorus in lake. Environmental Chemistry, 2003, 22(5): 485-489. [王晓蓉, 丁丽丽, 牛晓君等. 磷化氢在湖泊磷生物地球化学循环中的作用. 环境化学, 2003, 22(5): 485-489. DOI:10.3321/j.issn:0254-6108.2003.05.014] |
| [10] |
Wetzel RG ed. Limnology: Lake and river ecosystems. Gulf Professional Publishing, 2001.
|
| [11] |
Søndergaard M, Jensen JP, Jeppesen E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia, 2003, 506/507/508/509(1/2/3): 135-145. DOI:10.1023/b:hydr.0000008611.12704.dd |
| [12] |
Søndergaard M, Windolf J, Jeppesen E. phosphorus fractions and profiles in the sediment of shallow Danish lakes as related to phosphorus load, sediment composition and lake chemistry. Water Research, 1996, 30(4): 992-1002. DOI:10.1016/0043-1354(95)00251-0 |
| [13] |
Qi C, Fang JQ, Zhang LM et al. Composition and distribution of biodegradable compounds in phytoplankton-dominated zone of Lake Taihu. J Lake Sci, 2019, 31(4): 941-949. [祁闯, 方家琪, 张利民等. 太湖藻型湖区沉积物中生物易降解物质组成及分布规律. 湖泊科学, 2019, 31(4): 941-949. DOI:10.18307/2019.0423] |
| [14] |
Zhang YD, Yu JL, Su YL et al. Long-term changes of water quality in aquaculture-dominated lakes as revealed by sediment geochemical records in Lake Taibai (Eastern China). Chemosphere, 2019, 235: 297-307. DOI:10.1016/j.chemosphere.2019.06.179 |
| [15] |
Boström B, Andersen JM, Fleischer S et al. Exchange of phosphorus across the sediment-water interface. Hydrobiologia, 1988, 170: 229-244. DOI:10.1007/BF00024907 |
| [16] |
Rydin E. Potentially mobile phosphorus in Lake Erken sediment. Water Research, 2000, 34(7): 2037-2042. DOI:10.1016/s0043-1354(99)00375-9 |
| [17] |
Kalff J, Knoechel R. Phytoplankton and their dynamics in oligotrophic and eutrophic lakes. Annual Review of Ecology and Systematics, 1978, 9(1): 475-495. DOI:10.1146/annurev.es.09.110178.002355 |
| [18] |
Diovisalvi N, Bohn VY, Piccolo MC et al. Shallow lakes from the Central Plains of Argentina: An overview and worldwide comparative analysis of their basic limnological features. Hydrobiologia, 2015, 752(1): 5-20. DOI:10.1007/s10750-014-1946-x |
| [19] |
Ptacnik R, Lepistö L, Willén E et al. Quantitative responses of lake phytoplankton to eutrophication in Northern Europe. Aquatic Ecology, 2008, 42(2): 227-236. DOI:10.1007/s10452-008-9181-z |
| [20] |
Jensen JP, Jeppesen E, Olrik K et al. Impact of nutrients and physical factors on the shift from cyanobacterial to chlorophyte dominance in shallow Danish lakes. Canadian Journal of Fisheries and Aquatic Sciences, 1994, 51(8): 1692-1699. DOI:10.1139/f94-170 |
| [21] |
Søndergaard M, Jeppesen E, Kristensen P et al. Interactions between sediment and water in a shallow and hypertrophic lake: A study on phytoplankton collapses in Lake Søbygård, Denmark. Hydrobiologia, 1990, 191(1): 139-148. DOI:10.1007/bf00026048 |
| [22] |
Boros G, Søndergaard M, Takács P et al. Influence of submerged macrophytes, temperature, and nutrient loading on the development of redox potential around the sediment-water interface in lakes. Hydrobiologia, 2011, 665(1): 117-127. DOI:10.1007/s10750-011-0609-4 |
| [23] |
Xie LQ, Xie P, Tang HJ. Enhancement of dissolved phosphorus release from sediment to lake water by Microcystis blooms-an enclosure experiment in a hyper-eutrophic, subtropical Chinese lake. Environmental Pollution, 2003, 122(3): 391-399. DOI:10.1016/s0269-7491(02)00305-6 |
| [24] |
Madsen TV, Cedergreen N. Sources of nutrients to rooted submerged macrophytes growing in a nutrient-rich stream. Freshwater Biology, 2002, 47(2): 283-291. DOI:10.1046/j.1365-2427.2002.00802.x |
| [25] |
Søndergaard M, Johansson LS, Lauridsen TL et al. Submerged macrophytes as indicators of the ecological quality of lakes. Freshwater Biology, 2010, 55(4): 893-908. DOI:10.1111/j.1365-2427.2009.02331.x |
| [26] |
Phillips G, Willby N, Moss B. Submerged macrophyte decline in shallow lakes: What have we learnt in the last forty years?. Aquatic Botany, 2016, 135: 37-45. DOI:10.1016/j.aquabot.2016.04.004 |
| [27] |
Hall NS, Pearl HW. Vertical migration patterns of phytoflagellates in relation to light and nutrient availability in a shallow microtidal estuary. Marine Ecology Progress Series, 2011, 425: 1-19. DOI:10.3354/meps09031 |
| [28] |
Wang C, Fan XL, Wang PF et al. Flow characteristics of the wind-driven current with submerged and emergent flexible vegetations in shallow lakes. Journal of Hydrodynamics, 2016, 28(5): 746-756. DOI:10.1016/s1001-6058(16)60677-7 |
| [29] |
Horppila J, Nurminen L. Effects of submerged macrophytes on sediment resuspension and internal phosphorus loading in Lake Hiidenvesi (southern Finland). Water Research, 2003, 37(18): 4468-4474. DOI:10.1016/s0043-1354(03)00405-6 |
| [30] |
Li EH, Li W, Liu GH et al. The effect of different submerged macrophyte species and biomass on sediment resuspension in a shallow freshwater lake. Aquatic Botany, 2008, 88(2): 121-126. DOI:10.1016/j.aquabot.2007.09.001 |
| [31] |
Barko JW, James WF. Effects of submerged aquatic macrophytes on nutrient dynamics, sedimentation, and resuspension//Jeppesen E, Søndergaard M, Søndergaard M et al eds. The structuring role of submerged macrophytes in lakes. New York: Springer New York, 1998: 197-214. DOI: 10.1007/978-1-4612-0695-8_10.
|
| [32] |
Jensen M, Liu ZW, Zhang XF et al. The effect of biomanipulation on phosphorus exchange between sediment and water in shallow, tropical Huizhou West Lake, China. Limnologica, 2017, 63: 65-73. DOI:10.1016/j.limno.2017.01.001 |
| [33] |
Zhang XF, Liu ZW, Jeppesen E et al. Effects of benthic-feeding common carp and filter-feeding silver carp on benthic-pelagic coupling: Implications for shallow lake management. Ecological Engineering, 2016, 88: 256-264. DOI:10.1016/j.ecoleng.2015.12.039 |
| [34] |
Yan YJ, Li XY, Liang YL. A comparative study on community structure ofmacrozoobenthos between macrophtic and algal lakes. J Lake Sci, 2005, 17(2): 176-182. [闫云君, 李晓宇, 梁彦龄. 草型湖泊和藻型湖泊中大型底栖动物群落结构的比较. 湖泊科学, 2005, 17(2): 176-182. DOI:10.18307/2005.0214] |
| [35] |
Shu FY, Wang HJ, Pan BZ et al. Assessment of species status of Mollusca in the mid-lower Yangtze lakes. Acta Hydrobiologica Sinica, 2009, 33(6): 1051-1058. [舒凤月, 王海军, 潘保柱等. 长江中下游湖泊贝类物种濒危状况评估. 水生生物学报, 2009, 33(6): 1051-1058. DOI:10.3724/SP.J.0000.2009.61051] |
| [36] |
Pan BZ, Wang HZ, Pusch MT et al. Macroinvertebrate responses to regime shifts caused by eutrophication in subtropical shallow lakes. Freshwater Science, 2015, 34(3): 942-952. DOI:10.1086/682077 |
| [37] |
MacIsaac HJ. Filtering impacts of an introduced bivalve (Dreissena polymorpha) in a shallow lake: Application of a hydrodynamic model. Ecosystems, 1999, 2(4): 338-350. DOI:10.1007/s100219900084 |
| [38] |
Zhang XF, Liu ZW, Jeppesen E et al. Effects of deposit-feeding tubificid worms and filter-feeding bivalves on benthic-pelagic coupling: Implications for the restoration of eutrophic shallow lakes. Water Research, 2014, 50: 135-146. DOI:10.1016/j.watres.2013.12.003 |
| [39] |
Li Y, Cai YJ, Qin BQ et al. Temporal and spatial patterns of Limnodrilus hoffmeisteri claparède in Lake Taihu. J Lake Sci, 2012, 24(3): 450-459. [李艳, 蔡永久, 秦伯强等. 太湖霍甫水丝蚓(Limnodrilus hoffmeisteri Claparède)的时空格局. 湖泊科学, 2012, 24(3): 450-459. DOI:10.18307/2012.0318] |
| [40] |
Mo SQ, Zhang XF, Tang YL et al. Effects of snails, submerged plants and their coexistence on eutrophication in aquatic ecosystems. Knowledge & Management of Aquatic Ecosystems, 2017, 418: 44. DOI:10.1051/kmae/2017034 |
| [41] |
Yang L, He H, Guan BH et al. Mesocosm experiment reveals a strong positive effect of snail presence on macrophyte growth, resulting from control of epiphyton and nuisance filamentous algae: Implications for shallow lake management. Science of the Total Environment, 2020, 705: 135958. DOI:10.1016/j.scitotenv.2019.135958 |
| [42] |
Li KY, Liu ZW, Gu BH. Persistence of clear water in a nutrient-impacted region of Lake Taihu: The role of periphyton grazing by snails. Fundamental and Applied Limnology, 2008, 173(1): 15-20. DOI:10.1127/1863-9135/2008/0173-0015 |
| [43] |
He H, Liu XB, Liu XL et al. Effects of cyanobecterial blooms on submerged macrophytes alleviated by the native Chinese bivalve Hyriopsis cumingii: A mesocosm experiment study. Ecological Engineering, 2014, 71: 363-367. DOI:10.1016/j.ecoleng.2014.07.015 |
| [44] |
Vadeboncoeur Y, Zanden MJV, Lodge DM. Putting the lake back together: Reintegrating benthic pathways into lake food web models: Lake ecologists tend to focus their research on pelagic energy pathways, but, from algae to fish, benthic organisms form an integral part of lake food webs. Bioscience, 2002, 52(1): 44-54. DOI:10.1641/0006-3568(2002)052[0044:PTLBTR]2.0.CO;2 |
| [45] |
Zanden MJV, Vadeboncoeur Y. Fishes as integrators of benthic and pelagic food webs in lakes. Ecology, 2002, 83(8): 2152. DOI:10.2307/3072047 |
| [46] |
Jeppesen E, Jensen JP, Søndergaard M et al. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshwater Biology, 2000, 45(2): 201-218. DOI:10.1046/j.1365-2427.2000.00675.x |
| [47] |
Plew DR, Klebert P, Rosten TW et al. Changes to flow and turbulence caused by different concentrations of fish in a circular tank. Journal of Hydraulic Research, 2015, 53(3): 364-383. DOI:10.1080/00221686.2015.1029016 |
| [48] |
Lammens EHRR, Hoogenboezem W. Diets and feeding behavior// Winfield IJ, Nelson JS eds. Cyprinid fishes: Systematics, biology and exploitation. Chapman and Hall, 1991.
|
| [49] |
Breukelaar AW, Lammens EHRR, Breteler JGPK et al. Effects of benthivorous bream (Abramis brama) and carp (Cyprinus carpio) on sediment resuspension and concentrations of nutrients and chlorophyll a. Freshwater Biology, 1994, 32(1): 113-121. DOI:10.1111/j.1365-2427.1994.tb00871.x |
| [50] |
Weber MJ, Brown ML. Effects of common carp on aquatic ecosystems 80 years after "carp as a dominant": Ecological insights for fisheries management. Reviews in Fisheries Science, 2009, 17(4): 524-537. DOI:10.1080/10641260903189243 |
| [51] |
Huser BJ, Bajer PG, Chizinski CJ et al. Effects of common carp (Cyprinus carpio) on sediment mixing depth and mobile phosphorus mass in the active sediment layer of a shallow lake. Hydrobiologia, 2016, 763(1): 23-33. DOI:10.1007/s10750-015-2356-4 |
| [52] |
Scheffer M, Portielje R, Zambrano L. Fish facilitate wave resuspension of sediment. Limnology and Oceanography, 2003, 48(5): 1920-1926. DOI:10.4319/lo.2003.48.5.1920 |
| [53] |
Guo LG, Wang Q, Xie P et al. A non-classical biomanipulation experiment in Gonghu Bay of Lake Taihu: Control of Microcystis blooms using silver and bighead carp. Aquaculture Research, 2015, 46(9): 2211-2224. DOI:10.1111/are.12375 |
| [54] |
Jeppesen E, Jensen JP, Søndergaard M et al eds. Top-down control in freshwater lakes: The role of nutrient state, submerged macrophytes and water depth. Shallow Lakes '95. Dordrecht: Springer Netherlands, 1997: 151-164. DOI: 10.1007/978-94-011-5648-6_17
|
| [55] |
Jeppesen E, Jensen JP, Jensen C et al. The impact of nutrient state and lake depth on top-down control in the pelagic zone of lakes: A study of 466 lakes from the temperate zone to the arctic. Ecosystems, 2003, 6(4): 313-325. DOI:10.1007/pl00021503 |
| [56] |
Zeng HY, Zhong P, Zhao XF et al. Response of metazoan zooplankton communities to ecological restoration in a tropical shallow lake. J Lake Sci, 2016, 28(1): 170-177. [曾海逸, 钟萍, 赵雪枫等. 热带浅水湖泊后生浮游动物群落结构对生态修复的响应. 湖泊科学, 2016, 28(1): 170-177. DOI:10.18307/2016.0120] |
| [57] |
Chen FZ, Shu TT, Jeppesen E et al. Restoration of a subtropical eutrophic shallow lake in China: Effects on nutrient concentrations and biological communities. Hydrobiologia, 2013, 718(1): 59-71. DOI:10.1007/s10750-013-1603-9 |
| [58] |
Liu ZW, Hu JR, Zhong P et al. Successful restoration of a tropical shallow eutrophic lake: Strong bottom-up but weak top-down effects recorded. Water Research, 2018, 146: 88-97. DOI:10.1016/j.watres.2018.09.007 |
| [59] |
Zambrano L, Hinojosa D. Direct and indirect effects of carp (Cyprinus carpio L.) on macrophyte and benthic communities in experimental shallow ponds in central Mexico. Shallow Lakes '98. Dordrecht: Springer Netherland, 1999, 131-138. DOI:10.1007/978-94-017-2986-4_13 |
| [60] |
Yu JL, Liu ZW, Li KY et al. Restoration of shallow lakes in subtropical and tropical China: Response of nutrients and water clarity to biomanipulation by fish removal and submerged plant transplantation. Water, 2016, 8(10): 438. DOI:10.3390/w8100438 |
| [61] |
Søndergaard M, Jensen PJ, Jeppesen E. Retention and internal loading of phosphorus in shallow, eutrophic lakes. The Scientific World Journal, 2001(1): 427-442. DOI:10.1100/tsw.2001.72 |
 2020, Vol. 32
2020, Vol. 32 

