 、鳙、草鱼、鲢,华鳈、光唇蛇鮈、蛇鮈.通过单因素多元方差分析解析不同生境过滤条件下鱼类物种和功能多样性的差异,发现在物种多样性层面,沿岸带的Richness指数和Shannon-Wiener指数显著低于其他微生境;在功能多样性层面,不仅发现沿岸带显著较低的RaoQ指数,同时发现河道的FDiv指数显著高于其他微生境类型.
、鳙、草鱼、鲢,华鳈、光唇蛇鮈、蛇鮈.通过单因素多元方差分析解析不同生境过滤条件下鱼类物种和功能多样性的差异,发现在物种多样性层面,沿岸带的Richness指数和Shannon-Wiener指数显著低于其他微生境;在功能多样性层面,不仅发现沿岸带显著较低的RaoQ指数,同时发现河道的FDiv指数显著高于其他微生境类型.受河流洪泛周期性的影响,河流—泛滥平原生态系统具有复杂的生境异质性,是鱼类多样性最为丰富的区域之一[1-2].根据Ward[3]提出的河流—泛滥平原水文过程的4维特征,这种生境异质性可以拆分为时间维度下的纵向、横向和垂直异质性.其中,纵向维度是指河流上下游之间的差异,横向维度表征河流干流与临接湖泊以及附属水体的异质状况,垂直维度则指示地表水和地下水之间的差异[4].河流连续统理论(River Continuum Concept, RCC)[5]的形成,使得大量的学者探讨和研究河流上下游之间生境过滤作用对鱼类群落的影响.然而,针对通江湖泊等泛滥平原水域生境过滤对鱼类影响的研究相对较少.受河流洪泛的影响,河道、湖口、沿岸带和敞水区是泛滥平原物质、能量和生物交流的关键微生境斑块(habitat patch)[6].全面厘清鱼类通过生长、繁殖、迁移和分布等生理生态特征对泛滥平原生境异质性的响应,可以为泛滥平原生态系统的鱼类资源保护提供科学依据.
生境过滤作用(habitat filtering)是指某一类型生境通过气候、水文、植被条件等环境要素以及生态位的分化对具有该生境适应性的物种进行定向选择的过程,具有相似适应性功能性状的物种被筛选出来,其决定了群落中物种共存的过程和机制[7-8].研究生境过滤作用对于了解群落构建[9]、解释物种多样性变化规律[10]、探索性状分化与环境之间的关系以及指导生物多样性保护和修复[9]都有重要意义.一方面,泛滥平原微生境可以通过水体流速、水层空间等环境条件过滤具有适应性性状的鱼类:例如,河流生境过滤的鱼类往往有长而粗壮的尾柄方便抵抗水流、自主泳动,敞水区水体底层通过生境过滤筛选出的鱼类多长有须以补偿光线的不足[11-15].另一方面,泛滥平原微生境通过提供产卵场或饵料条件对鱼类的迁移与洄游进行定向选择:产漂流性卵的鱼类周期性地选择河道、湖泊生境进行产卵、育肥[16-17],而产粘性卵的鱼类则在水草密布的沿岸带产卵以及在开阔的敞水区越冬和避难[18].泛滥平原生态系统不同微生境在水文连通性的支持下为鱼类的生存和繁衍创造了条件[19],微生境斑块的时空异质性对鱼类物种多样性的维持具有重要意义.
惯常的生境过滤作用对鱼类群落影响的研究基于鱼类物种组成和多样性,没有考虑鱼类的功能性状,然而微生境主要通过鱼类功能性状过滤物种[20].基于功能性状的功能多样性被认为是有效解析群落和环境相互作用关系的有力工具[21-23],先前的研究证实了功能多样性可以更好的阐明生境过滤背景下的群落集群规律[24-25],方便我们更好地解析物种—环境互作的生态系统过程[26-29].本研究以长江中下游平原地区的菜子湖流域为例,选择沿岸带、敞水区、湖口和河道4种典型微生境类型设置样点进行鱼类的定量调查采集,在充分考虑物种和功能多样性的基础上,全面探讨生境过滤作用对鱼类群落的影响,为泛滥平原鱼类多样性的维持提供科学依据.
1 材料与方法 1.1 研究区域概况及鱼类的调查采集菜子湖(30°43′~30°58′N,117°01′~117°09′E)位于长江北岸安徽省境内,是长江下游典型泛滥平原湖泊[30].菜子湖流域属于亚热湿润季风气候,年平均气温16.6℃,年平均降水量1325.5 mm[31].菜子湖水位变化显著,每年5—10月为丰水期,7—8月达到最高水位,丰水期水深最高可达7 m;11月至次年4月为枯水期,水位明显下降,最低处水深仅80 cm;菜子湖丰水期面积可达242.3 km2,平水期为172.1 km2,枯水期面积仅为145.2 km2[32-34].湖区鱼类资源丰富,已记录的鱼类共有8目18科68种,主要的经济鱼类有青鱼、草鱼、鲢、鳙、团头鲂和鲫等[32].
为了排除季节因子对生境过滤的干扰且考虑到丰水期鱼类较好的可获得性,本研究集中于2018年6—8月在菜子湖“沿岸带”、“敞水区”、“湖口”以及菜子湖与长江连通的长河“河道”4类生境分别设置12个样点进行鱼类的定量调查采集.其中,“沿岸带”选择菜子湖湖周水深1~2 m的近岸水域设置样点,“敞水区”选择水深超过3 m的湖心水域设置样点,“湖口”选取菜子湖与长河连通的回水区设置样点,而“河道”生境的所有样点分布于长河不同断面.结合当地渔民惯常的渔业作业水域,4类微生境各样点尽可能随机均匀分布.为了确保数据的横向可比性,各样点均使用网目和规格相同的网簖进行作业.网簖由网目为30 mm的聚乙烯网片制作而成,由引网、围网和网袋3部分组成[35],每组网簖的引网长75 m,围网面积约为50 m2,网袋2个,引网和围网均高于水面50 cm.于前一天下午置网至第二天清晨取鱼,超过12 h过夜捕捞,CPUE统计为单网渔获量.在实际采样过程中,因不可抗力因素部分样点没有采到鱼类,沿岸带、敞水区、湖口、河道分别采集了10、12、10和11个样点的数据.鱼类样品采集完成后,当场鉴定到种,测量体长、体重,并记录.使用量鱼板测量体长,精确到1 mm;电子秤称量体重,精确到0.1 g.若无法当场鉴定到种,测量、称重、记录完成后,使用10 %的福尔马林溶液浸泡鱼类样品制成标本带回实验室鉴定.
1.2 数据处理与分析方法 1.2.1 多样性测度本研究选取Richness指数、Shannon-Wiener指数和Pielou指数表征不同微生境条件下鱼类群落的物种多样性.同时,选择RaoQ指数(Quadratic Entropy),FDiv指数(Functional divergence)和FEve指数(Functional evenness)表征不同微生境条件下鱼类群落的功能多样性.
功能多样性指数基于“样点×物种”和“物种×功能性状”的矩阵进行计算.其中,功能性状选取最大体长、体型(侧扁形、平扁形、圆筒形、纺锤形、卵圆形、鳗形)、生态类型(湖泊定居性、山溪河流性、江湖洄游性)、食性(植食性、杂食性、底栖生物食性、碎屑食性、肉食性、浮游生物食性)、生长速率、寿命、初次性成熟时间、初次性成熟体长、营养级9个生态学参数进行表征.功能性状的选择依据、度量标准和具体参数沿用本团队已发表的菜子湖流域功能群的研究[36].
RaoQ指数通过物种功能性状计算的距离矩阵和物种相对丰度向量解释一个群落中物种的平均距离差异[37],计算公式为:
| $ \operatorname{RaoQ}=\sum\limits_{i=1}^{S-1} \sum\limits_{j=i+1}^{S-1} d_{i j} p_{i} p_{j} $ | (1) |
式中,S为物种数,dij为物种i和j在系统发育树上的离散距离,pi为物种i的相对丰度,pj为物种j的相对丰度.
FDiv指数可以用来解释物种丰度在功能性状空间的散布情况,度量每个物种与功能上趋异化物种质心的平均距离[38],计算公式为:
| $ g_{k}=\frac{1}{S} \sum\limits_{i=1}^{S} x_{i k} $ | (2) |
| $ d G_{i}=\sqrt{\sum\nolimits_{k=1}^{T}\left(x_{i k}-g_{k}\right)^{2}} $ | (3) |
| $ \overline{d G}=\frac{1}{S} \sum\limits_{i=1}^{S} d G_{i} $ | (4) |
| $ \Delta d=\sum\limits_{i=1}^{s} w_{i}\left(d G_{i}-\overline{d G}\right) $ | (5) |
| $ \Delta|d|=\sum\limits_{i=1}^{s} w_{i}\left|d G_{i}-\overline{d G}\right| $ | (6) |
| $\text { FDiv }=\frac{\Delta d+\overline{d G}}{\Delta|d|+\overline{d G}} $ | (7) |
式中,xik为物种i性状k的值,gk为性状k的中心,T为性状数量,dG为物种i与重心的平均距离,d为以多度为权重的离散度,wi为物种i的相对多度.
FEve指数在要求每个样点的物种数不少于3个的条件下,使用最小生成树(MST)的方法计算物种功能性状分布的均匀性和物种丰度分布的均匀性[38],计算公式为:
| $ \text { FEve }=\frac{\sum\limits_{L=1}^{S-1} \min \left(P E W_{L}, \frac{1}{S-1}\right)-\frac{1}{S-1}}{1-\frac{1}{S-1}} $ | (8) |
| $ P E W_{L}=\frac{E W_{L}}{\sum\limits_{L=1}^{S-1} E W_{L}} $ | (9) |
| $ E W_{L}=\frac{d i s t(i, j)}{w_{i}+w_{j}} $ | (10) |
式中,S为物种数,EWL为均匀度权重;dist(i, j)为物种i和j的欧式距离;wi为物种i的相对丰富度;L为分支长;PEWL为分支长权重.
1.2.2 统计分析本研究首先将群落物种组成数据进行lg(x+1)转化,在此基础上构建了Bray-Curtis距离矩阵;而后采用单因素多元方差分析(One-way PERMANOVA)比较沿岸带、敞水区、湖口和河道4种微生境下鱼类群落组成是否存在差异;在差异显著的条件下,进一步通过多维标度排序分析(MDS)筛选出引起群落组成差异的代表种(Correlation>0.7).同时,通过单因素多元方差分析(One-way PERMANOVA)解析不同生境过滤条件下菜子湖流域鱼类物种和功能多样性的差异.
物种多样性指数及One-way PERMANOVA分析采用PAST软件进行计算,MDS分析使用Primer 6和Permanova+软件[39-40],在R studio中用FD包[41]计算功能多样性指数,用Origin 9.0软件进行绘图.
2 结果 2.1 物种组成沿岸带、敞水区、湖口、河道4种微生境共采集鱼类5194尾,隶属5目31属43种,以鲤形目(Cypriniformes)种类最多,共29种,占总数的67.4 %;鲱形目(Clupeiformes)1种,占总数的2.3 %;鲶形目(Siluriformes)5种,占总数的11.6 %;颌针鱼目(Beloniformes)1种,占总数的2.3 %;鲈形目(Perciformes)7种,占总数的16.3 %.其中,沿岸带采集到的鱼类隶属5目28属34种;敞水区采集的鱼类隶属5目31属42种;湖口采集的鱼类隶属5目31属43种;河道采集的鱼类隶属5目30属41种(附录Ⅰ).
| 附录Ⅰ 菜子湖沿岸带、敞水区、湖口以及河道4种微生境鱼类名录 Appendix Ⅰ List of fish species in littoral zone, open water, lake inlets and river channel of Lake Caizi |
根据泛滥平原生态系统鱼类的生活史周期和栖息地偏好特征,可以将本研究鱼类的生态类型划分为湖泊定居性鱼类、山溪河流性鱼类和江湖洄游性鱼类3大类.湖泊定居性鱼类的物种数在4种微生境条件下的变化不大(沿岸带26种,敞水区29种,湖口30种,河道28种),但河道生境中湖泊定居性鱼类的个体数和百分比明显少于其他3种微生境类型(表2).同时,河道生境中山溪河流性鱼类的个体数和百分比明显高于其他3种生境类型(河道148尾、12.97 %;沿岸带79尾、8.20 %;敞水区52尾、2.88 %;湖口67尾、5.23 %),而沿岸带的山溪河流性鱼类物种数明显少于其他3种微生境类型(沿岸带3种,敞水区6种,湖口6种,河道6种).此外,敞水区江湖洄游性鱼类的个体数和百分比较高(438尾、24.21 %),湖口、河道以及敞水区江湖洄游性鱼类的物种数差异不大,但均高于沿岸带(表 1).
| 表 1 沿岸带、敞水区、湖口、河道4种微生境鱼类的生态类型 Tab. 1 The ecological types of fish species in littoral zone, open water, lake inlets and river channel |
通过对菜子湖4种微生境类型下鱼类群落的单因素多元方差分析(One-way PERMANOVA),发现沿岸带、敞水区、湖口和河道4种微生境条件下的鱼类群落有显著差异(F=4.889, P < 0.0001).进一步通过多维标度排序(MDS)分析显示,4种微生境内的鱼类区系可分为3组,沿岸带、敞水区和河道可以在排序轴上很好地区分开(图 1).沿岸带丰度较高的代表种为

|
图 1 沿岸带、敞水区、湖口和河道4种微生境下鱼类群落的多维尺度排序 Fig.1 The multi-dimensional scaling(MDS) of fish communities in littoral zone, open water, lake inlets and river channel |
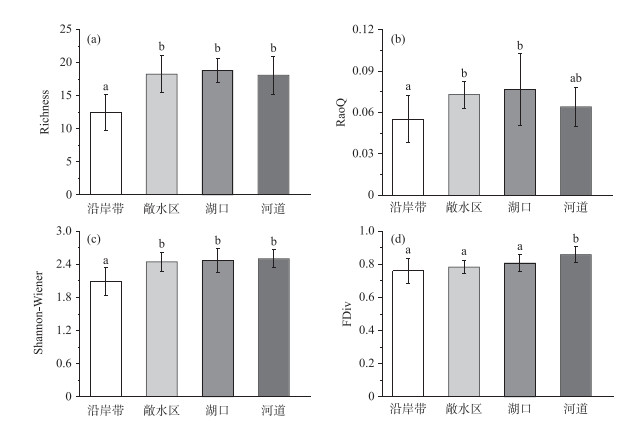
通过One-way PERMANOVA分析比较菜子湖4种微生境条件下鱼类群落物种和功能多样性的差异,发现Richness指数、Shannon-Wiener指数、RaoQ指数和FDiv指数有显著差异(图 2).其中,沿岸带的Richness指数和Shannon-Wiener多样性指数均显著低于敞水区、湖口以及河道(P < 0.05)(图 2a、c).同时,沿岸带的RaoQ指数显著低于敞水区和湖口(P < 0.05)(图 2b).河道的FDiv指数显著高于沿岸带、敞水区和湖口(P < 0.05)(图 2d). Pielou均匀性指数和FEve指数在4种微生境条件下不显著(P>0.05).
|
图 2 沿岸带、敞水区、湖口和河道4种微生境鱼类的物种多样性指数和功能多样性指数 (不同的上标字母表示有显著差异,P < 0.05) Fig.2 The diversity indexes as well as functional indexes of fish species in littoral zone, open water, lake inlets and river channel (Different superscript letters are significantly different, P < 0.05) |
泛滥平原生态系统通过微生境的异质性为鱼类提供栖息、产卵、索饵和越冬的场所,从而提高鱼类的存活率并进一步保证物种的延续[19, 42].此外,基于索饵、繁殖、越冬等生理生态需求,在通江湖泊水域连通的支持下,鱼类需要在不同生境斑块间迁移和洄游完成其生活史周期和节律[43-44].然而,在围湖造田、围网养殖、闸坝修建等人为干扰的影响下,泛滥平原生态系统生境斑块的种类和数量正在逐步退化[45].同时,不合理的水文阻隔和水资源调蓄改变了泛滥平原的自然连通性,进而造成微生境的同质化,这在一定程度上对鱼类多样性产生了不利的影响[46-47].在此背景下,全面厘清泛滥平原生境过滤作用对鱼类物种和功能组成的影响十分必要.
泛滥平原微生境异质性在一定程度上影响鱼类生态习性的适应性[24],进一步决定鱼类物种组成的差异[48].目前已有的研究表明泛滥平原湖泊沿岸带生态幅较小,生态稳定性较差,难以支撑较高的鱼类多样性[49].本研究得到了类似的结果,相对于敞水区、湖口和河道采集到的鱼类物种(42、43和41种),沿岸带的物种数最低(34种).菜子湖隶属长江流域吞吐型湖泊,沿岸带随水位周期性变化,且易受到围网养殖、水体污染等人类活动的干扰,难以维持较高的鱼类多样性[49].河道微生境中山溪河流性鱼类的个体数和百分比明显高于沿岸带、敞水区和湖口(河道148尾、12.97 %;沿岸带79尾、8.20 %;敞水区52尾、2.88 %;湖口67尾、5.23 %),并且其江湖洄游性鱼类与湖泊定居性鱼类物种数与湖口、敞水区类似,均明显高于沿岸带.河道作为大部分鱼类产卵和繁殖的场所,具备丰富的生态功能和复杂的水文、底质环境,能够支撑更高的物种多样性[50],同时高的水文连通性使其拥有更广的生态幅以容纳多种生态类型的鱼类[19].
在生境过滤的作用下,泛滥平原生态系统不同微生境会筛选出适应该生境条件的鱼类代表物种[51-52].基于此,本研究在One-way PERMANOVA分析表明菜子湖沿岸带、敞水区、湖口和河道4种微生境下鱼类群落有显著差异(F = 4.889, P < 0.0001)的条件下,探讨了河道、敞水区及沿岸带丰度较高的代表物种.其中,河道丰度较高的代表种为华鳈、光唇蛇鮈和蛇鮈:均属栖息于流水水体中下层,游泳能力较强的小型鱼类,广布于长江水系[53-54].敞水区丰度较高的代表种为鳙、草鱼和鲢:属于大型淡水鱼类,需要敞水区稳定的水文环境和丰富的饵料资源保证其快速生长[17].沿岸带丰度较高的代表种为
生境过滤作用的实质是通过特定微生境环境将具有相同或相似功能性状的物种集群,进行定向选择的过程[56-57].其不仅能够筛选和刻画出鱼类的物种多样性组成,更重要的是能决定鱼类功能多样性差异[24].本研究发现,在物种多样性方面,沿岸带的Richness指数、Shannon-Wiener指数与河道、敞水区和湖口相比均显著降低(P < 0.05).在洪泛周期[48],围湖造田[58]、水体污染[59]等干扰因素的背景下,沿岸带生境的不稳定性和脆弱性导致了其相对较低的物种和功能多样性.功能多样性指数比对的结果不仅指示出沿岸带的RaoQ指数最低、功能较为单一,还展示出河道的FDiv指数显著高于其他3种微生境(P < 0.05),这表明河道的鱼类在功能上较其他3种微生境更为趋异,包含更多功能独特的物种.复杂的微生境异质性,丰富的生态功能以及与高的水文连通性使河道微生境能够容纳更高的功能多样性[19, 50].与物种多样性相比,功能多样性不仅表征了沿岸带同时也反映了河道微生境的生境过滤作用,因而展现出更高的敏感性[60-64].
泛滥平原生态系统微生境多样性不仅支撑了鱼类的物种多样性[65], 并且对于生态系统功能和干扰后的生态恢复都有着重要意义[66].微生境的多样性不仅提供鱼类生存繁衍的重要环境条件,也是决定物种分布和迁移的决定性因素[67].然而,当前频繁的人为干扰对泛滥平原生态系统原有的生境异质性造成了破坏.例如,闸坝修筑引发的江湖阻隔,减少了各个微生境之间的水文连通性,导致微生境同质化,使一部分洄游性鱼类无法完成在不同微生境间的迁移行为[68];湖泊围垦导致的湖泊面积侵蚀,同时会对沿岸带微生境产生剧烈的影响[69];各种工业点源和农业面源污染通过地表径流经沿岸带流入或者随河流水文周期进入泛滥平原,对鱼类生存和繁衍造成严重威胁的同时会对鱼类多样性产生不利影响[70].因此,有必要从维持自然的水文连通[71]、防止生境退化[72]、减少水体污染[73]等方面着手:严禁在湖内筑堤垦殖,取缔围湖造田等侵蚀行为,保护易受干扰的沿岸带生境;加强菜子湖与长江的水文联系,尤其在鱼苗入湖和亲鱼归江时保证江湖的自然连通,使鱼类能够通过在微生境间的迁移完成其正常的生活史周期和节律,确保物种的延续;严控工业废水和生活污水的排放,防止水体污染.进而全面维持泛滥平原微生境的连通性、多样性和生态系统健康,有效保护泛滥平原鱼类资源.
| [1] |
Dudgeon D, Arthington AH, Gessner MO et al. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews, 2006, 81: 163-182. DOI:10.1017/S1464793105006950 |
| [2] |
Röpke CP, Amadio SA, Winemiller KO et al. Seasonal dynamics of the fish assemblage in a floodplain lake at the confluence of the Negro and Amazon Rivers. Journal of Fish Biology, 2016, 89: 194-212. DOI:10.1111/jfb.12791 |
| [3] |
Ward JV. Riverine-wetland interactions// Sharitz RR, Gibbons JW eds. Freshwater wetlands and wildlife. Oak Ridge, Tennessee, USA: United States Department of Energy, 1989: 385-400.
|
| [4] |
Zhang L, Pan BZ, Jiang XM et al. Research progress on the river-lake relation based on hydrological connectivity analysis. Resources and Environment in the Yangtze Basin, 2018, 27: 2805-2816. [张磊, 潘保柱, 蒋小明等. 基于水文连通分析的江湖关系研究进展. 长江流域资源与环境, 2018, 27: 2805-2816. DOI:10.11870/cjlyzyyhj201812017] |
| [5] |
Vannote RL, Minshall GW, Cummins KW et al. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences, 1980, 37: 130-137. DOI:10.1139/f80-017 |
| [6] |
Thomaz SM, Bini LM, Bozelli RL. Floods increase similarity among aquatic habitats in river floodplain systems. Hydrobiologia, 2007, 579: 1-13. DOI:10.1007/s10750-006-0285-y |
| [7] |
van der Valk AG. Succession in wetlands: A gleasonian appraoch. Ecology, 1981, 62(3): 688. DOI:10.2307/1937737 |
| [8] |
Cornwell WK, Schwilk DW, Ackerly DD. A trait-based test for habitat filtering: convex hull volume. Ecology, 2006, 87: 1461-1471. |
| [9] |
Cadotte MW, Tucker CM. Should environmental filtering be abandoned?. Trends in Ecology and Evolution, 2017, 32: 429-437. DOI:10.1016/j.tree.2017.03.004 |
| [10] |
Laliberté EG, Zemunik, Turner BL. Environmental filtering explains variation in plant diversity along resource gradients. Science, 2014, 345(6204): 1602-1605. DOI:10.1126/science.1256330 |
| [11] |
Watson DJ, Balon EK. Ecomorphological analysis of fish taxocenes in rainforest streams of northern Borneo. Journal of Fish Biology, 1984, 25: 371-384. DOI:10.1111/j.1095-8649.1984.tb04885.x |
| [12] |
Winemiller KO. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecological Monographs, 1991, 16: 343-365. DOI:10.2307/2937046 |
| [13] |
Gatz AJ. Ecological morphology of freshwater stream fishes. Tulane Studies in Zoology and Botany, 1979, 21(2): 91-124. |
| [14] |
Sibbing FA, Nagelkerke LAJ. Resource partitioning by Lake Tana barbs predicted from fish morphometrics and prey characteristics. Reviews in Fish Biology and Fisheries, 2001, 10: 393-437. DOI:10.1023/A:1012270422092 |
| [15] |
Fahr J, Kalko EKV. Biome transitions as centres of diversity: habitat heterogeneity and diversity patterns of West African bat assemblages across spatial scales. Ecography, 2011, 34(2): 177-195. DOI:10.1111/j.1600-0587.2010.05510.x |
| [16] |
Bayley PB. Factors affecting growth rates of young tropical floodplain fishes: seasonality and density-dependence. Environmental Biology of Fishes, 1988, 21(2): 127-142. DOI:10.1007/BF00004848 |
| [17] |
Xie P. Biodiversity crisis in the Yangtze River from point views of evolution and ecology. Beijing: Science Press, 2018: 26-38. [谢平. 从历史起源和现代生态透视长江的生物多样性危机. 北京: 科学出版社, 2018: 26-38.]
|
| [18] |
Ding F. Study on spawning field of fish spawning on plants in Dahuchi Lake using remote sensing technique[Dissertation]. Shanghai: Shanghai Ocean University, 2017. [丁放.利用遥感技术测定鄱阳湖大湖池草上产卵鱼类产卵场的研究[学位论文].上海: 上海海洋大学, 2017. http://cdmd.cnki.com.cn/Article/CDMD-10264-1017854606.htm ]
|
| [19] |
Couto TBD, Zuanon J, Olden JD et al. Longitudinal variability in lateral hydrologic connectivity shapes fish occurrence in temporary floodplain ponds. Canadian Journal of Fisheries and Aquatic Sciences, 2018, 75: 319-328. DOI:10.1139/cjfas-2016-0388 |
| [20] |
Poff LR, Nelson-Baker K. Habitat heterogeneity and algal-grazer interactions in streams: Explorations with a spatially explicit model. Journal of the North American Benthological Society, 1997, 16(1): 263-276. DOI:10.2307/1468256 |
| [21] |
Chapin FS, Zavaleta ES, Eviner VT et al. Consequences of changing biodiversity. Nature, 2000, 405: 234-242. DOI:10.1038/35012241 |
| [22] |
Naeem S, Wright JP. Disentangling biodiversity effects on ecosystem functioning: Deriving solutions to a seemingly insurmountable problem. Ecology Letters, 2003, 6: 567-579. DOI:10.1046/j.1461-0248.2003.00471.x |
| [23] |
Tilman D, Knops J, Wedin D et al. The influence of functional diversity and composition on ecosystem processes. Science, 1997, 277: 1300-1302. DOI:10.1126/science.277.5330.1300 |
| [24] |
Fichaux M, Béchade B, Donald J et al. Habitats shape taxonomic and functional composition of Neotropical ant assemblages. Oecologia, 2019, 189: 501. DOI:10.1007/s00442-019-04341-z |
| [25] |
Cunha ER, Winemiller KO, da Silva JCB et al. α and β diversity of fishes in relation to a gradient of habitat structural complexity supports the role of environmental filtering in community assembly. Aquatic Sciences, 2019, 81: 38. DOI:10.1007/s00027-019-0634-3 |
| [26] |
Bellwood DR, Wainwright PC, Fulton CJ et al. Assembly rules and functional groups at global biogeographical scales. Functional Ecology, 2002, 16: 557-562. DOI:10.1046/j.1365-2435.2002.00655.x |
| [27] |
Götzenberger L, Bello F, Brathen KA et al. Ecological assembly rules in plant communities-approaches, patterns and prospects. Biological Reviews, 2012, 87: 111-127. DOI:10.1111/j.1469-185X.2011.00187.x |
| [28] |
Hoeinghaus DJ, Winemiller KO, Birnbaum JS. Local and regional determinants of stream fish assemblages structure: Inferences based on taxonomic vs. functional groups. Journal of Biogeography, 2007, 34: 324-338. DOI:10.1111/j.1365-2699.2006.01587.x |
| [29] |
Mason NWH, Lanoiselée C, Mouillot D et al. Does niche overlap control relative abundance in French lacustrine fish communities? A new method incorporating functional traits. Journal of Animal Ecology, 2008, 77: 661-669. DOI:10.1111/j.1365-2656.2008.01379.x |
| [30] |
Liu XH. Annual dynamics of phytoplankton abundance and community structure (2010) in Lake Caizi, Anhui Province. J Lake Sci, 2012, 24(5): 771-779. [刘雪花. 安徽菜子湖浮游植物群落结构的周年变化(2010年). 湖泊科学, 2012, 24(5): 771-779. DOI:10.18307/2012.0519] |
| [31] |
Ge Y, Guo M, Cao YY et al. Insect community diversity of different habitats in Caizihu wetland at the middle and lower reaches of the Yangtze River. Chinese Journal of Ecology, 2014, 33(8): 2084-2090. [葛洋, 郭苗, 曹玉言等. 长江中下游菜子湖湿地不同生境昆虫群落多样性. 生态学杂志, 2014, 33(8): 2084-2090. DOI:10.13292/j.1000-4890.2014.0190] |
| [32] |
Wang CX, Wang ZS, Xu LJ et al. Dynamics of fish community structure in Lake Caizi and its driving factors. Journal of Capital Normal University: Natural Science Edition, 2012, 33(4): 32-37. [王晨旭, 王忠锁, 许隆君等. 菜子湖鱼类区系变动及其驱动力分析. 首都师范大学学报:自然科学版, 2012, 33(4): 32-37.] |
| [33] |
Xu XY, Zhou LZ, Zhu WZ et al. Community structure of macrozoobenthos in Caizi Lake, China. Acta Ecologica Sinica, 2010, 31(4): 943-953. [徐小雨, 周立志, 朱文中等. 安徽菜子湖大型底栖动物的群落结构特征. 生态学报, 2010, 31(4): 943-953.] |
| [34] |
Peng J, Zhang PJ. The changes of soil organic matter contents in wetlands recovered from farmlands in Caizi Lake region. Yunnan Geographic Environment Research, 2012, 24(4): 92-96. [彭江, 张平究. 退耕还湖后菜子湖湿地土壤有机质含量变化. 云南地理环境研究, 2012, 24(4): 92-96. DOI:10.3969/j.issn.1001-7852.2012.04.017] |
| [35] |
Li W, Lin ML, Lian YX. The catch structure characteristics of Siniperca chuatsi captured by five fishing gears and its impact on Siniperca chuatsi resources in Xiaosihai Lake. Journal of Fisheries of China, 2015, 39(5): 712-719. [李为, 林明利, 连玉喜等. 肖四海湖五种渔具的鳜渔获结构特征及其对鳜资源的影响. 水产学报, 2015, 39(5): 712-719. DOI:10.11964/jfc.20140809415] |
| [36] |
Wang C, Jiang ZG, Zhou LZ et al. A functional group approach reveals important fish recruitments driven by flood pulses in floodplain ecosystem. Ecological Indicators, 2019, 99: 130-139. DOI:10.1016/j.ecolind.2018.12.024 |
| [37] |
Rao CR. Diversity and dissimilarity coefficients: A unified approach. Theoretical Population Biology, 1982, 21(1): 24-43. DOI:10.1016/0040-5809(82)90004-1 |
| [38] |
Villéger S, Mason NW, Mouillot D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology, 2008, 89: 2290-2301. DOI:10.1890/07-1206.1 |
| [39] |
Clarke KR, Gorley RN. PRIMER v6: User manual/tutorial. Primer-E, Plymouth, 2006.
|
| [40] |
Anderson MJ, Gorley RN, Clarke KR. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. Plymouth, UK: PRIMER-E, 2008.
|
| [41] |
Laliberté E, Legendre P, Shipley B. FD package: measuring functional diversity (FD) from multiple traits, and other tools for functional ecology, 2014. (https://cran.r-project.org/web/packages/FD/index.html)
|
| [42] |
Gomes LC, Agostinho AA. Influence of the flooding regime on the nutritional state and juvenile recruitment of the curimba, Prochilodus scrofa, Steindachner, in upper Parana River, Brazil. Fisheries Management & Ecology, 2010, 4(4): 263-274. DOI:10.1046/j.1365-2400.1997.00119.x |
| [43] |
Wang SY, Yang WJ, Huang MH et al. Fish passage and habitat restoration techniques in China: state of the art. Journal of Yangtze River Scientific Research Institute, 2017, 34(8): 11-17. [王思莹, 杨文俊, 黄明海等. 我国鱼类洄游通道和生境恢复技术研究现状分析. 长江科学院院报, 2017, 34(8): 11-17. DOI:10.11988/ckyyb.20160371] |
| [44] |
Winemiller KO, McIntyre PB, Castello L et al. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science, 2016, 351(6269): 128-129. DOI:10.1126/science.aac7082 |
| [45] |
Tockner K, Stanford JA. Riverine flood plains: present state and future trends. Environmental Conservation, 2002, 29: 308-330. DOI:10.1017/s037689290200022x |
| [46] |
Galat DL, Zueimüller I. Conserving large-river fishes: Is the highway analogy an appropriate paradigm?. Journal of the North American Benthological Society, 2001, 20: 266-279. DOI:10.2307/1468321 |
| [47] |
Nilsson C, Reidy CA, Dynesius M et al. Fragmentation and flow regulation of the World's large river systems. Science, 2005, 308: 405-408. DOI:10.1126/science.1107887 |
| [48] |
Tokeshi M, Arakaki S. Habitat complexity in aquatic systems: fractals and beyond. Hydrobiologia, 2012, 685: 27-47. DOI:10.1007/s10750-011-0832-z |
| [49] |
Cefalì ME, Cebrian E, Chappuis E et al. Life on the boundary: Environmental factors as drivers of habitat distribution in the littoral zone. Estuarine Coastal & Shelf Science, 2016, 172: 81-92. DOI:10.1016/j.ecss.2016.01.043 |
| [50] |
Naus CJ, Adams SR. Fish nursery habitat function of the main channel, floodplain tributaries and oxbow lakes of a medium-sized river. Ecology of Freshwater Fish, 2018, 27: 4-18. DOI:10.1111/eff.12319 |
| [51] |
Jackson DA, Somers KM, Harvey HH. Null models and fish communities: evidence of nonrandom patterns. American Naturalist, 1992, 139: 930-951. DOI:10.1086/285367 |
| [52] |
Kirschenbaum R, Grace JK. Dominant ant species in four habitats in Hawaii (Hymenoptera: Formicidae). Sociobiology, 2007, 50: 1069-1073. DOI:10.1590/S0085-56262007000100020 |
| [53] |
Dan Y, Ding B, Liu H. Isolation and characterization of 11 polymorphic microsatellite loci for the Saurogobio dabryi (Teleostei: Cyprinidae). Conservation Genetics Resources, 2009, 1(1): 471. DOI:10.1007/s12686-009-9109-5 |
| [54] |
Xuan YF, Wang RQ, Xu HH et al. Preliminary study on domestication culture experiment of Sarcocheilichthys sinensis. Scientific Fish Farming, 2010, 12: 32-32. [宣云峰, 王荣泉, 徐海华等. 华鳈驯化养殖试验初探. 科学养鱼, 2010, 12: 32-32.] |
| [55] |
Wang XL, Xiang JG, Liu JS et al. Reduced growth and reproductive investment of Hemiculter leucisculus (Cyprinidae) in a reservoir with introduced icefish Neosalanx taihuensis (Salangidae). Environmental Biology of Fishes, 2013, 96: 895-903. DOI:10.1007/s10641-012-0085-3 |
| [56] |
Bazzaz FA. Habitat selection in plants. The American Naturalist, 1991, 137: 116-130. DOI:10.1086/285142 |
| [57] |
Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology, 2002, 16: 545-556. DOI:10.1046/j.1365-2435.2002.00664.x |
| [58] |
Arèvalo R, Pinedo S, Ballesteros E. Changes in the composition and structure of Mediterranean rocky-shore communities following a gradient of nutrient enrichment: descriptive study and test of proposed methods to assess water quality regarding macroalgae. Mar Pollut Bull, 2007, 55: 104-113. DOI:10.1016/j.marpolbul.2006.08.023 |
| [59] |
Benedetti-Cecchi L, Bulleri F, Cinelli F. The interplay of physical and biological factors in maintaining mid-shore and low-shore assemblages on rocky coasts in the north-west Mediterranean. Oecologia, 2000, 123: 406-417. DOI:10.2307/4222634 |
| [60] |
Reich PB, Wright IJ, Cavender-Bares et al. The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Sciences, 2003, 164(S3): S143-S164. DOI:10.1086/374368 |
| [61] |
Gerisch M, Agostinelli V, Henle K et al. More species, but all do the same: contrasting effects of flood disturbance on ground beetle functional and species diversity. Oikos, 2012, 121: 508-515. DOI:10.1111/j.1600-0706.2011.19749.x |
| [62] |
Mouillot D, Graham NAJ, Villéger S et al. A functional approach reveals community responses to disturbances. Trends in Ecology & Evolution, 2013, 28: 167-177. DOI:10.1016/j.tree.2012.10.004 |
| [63] |
Toussaint A, Charpin N, Brosse S et al. Global functional diversity of freshwater fish is concentrated in the Neotropics while functional vulnerability is widespread. Scientific Reports, 2016, 6: 22125. DOI:10.1038/srep22125 |
| [64] |
Córdova-Tapia F, Zambrano L. The role of environmental filtering in the functional structure of fish communities in tropical wetlands. Ecology of Freshwater Fish, 2018, 27: 522-532. DOI:10.1111/eff.12366 |
| [65] |
Verhoef HA, Morin PJ. Community ecology: Processes, models and applications. London: Oxford University Press, 2010, 45-54. |
| [66] |
Dronova I. Environmental heterogeneity as a bridge between ecosystem service and visual quality objectives in management, planning and design. Landsc Urban Plan, 2017, 163: 90-106. DOI:10.1016/j.landurbplan.2017.03.005 |
| [67] |
Holderegger R, Wagner HH. Landscape genetics. BioScience, 2008, 58: 199-207. DOI:10.1641/B580306 |
| [68] |
Pringle C. What is hydrologic connectivity and why is it ecologically important?. Hydrol Process, 2003, 17: 2685-2689. DOI:10.1002/hyp.5145 |
| [69] |
Chang JB, Cao WX. Fishery significance of the river-communicating lakes and strategies for the management of fish resources. Resources and Environment in the Yangtze Basin, 1999, 8(2): 153-157. [常剑波, 曹文宣. 通江湖泊的渔业意义及其资源管理对策. 长江流域资源与环境, 1999, 8(2): 153-157. DOI:10.1007/978-94-011-3988-5_14] |
| [70] |
Silva SVS, Dias AHC, Dutra ES et al. The impact of water pollution on fish species in southeast region of Goiás, Brazil. Journal of Toxicology and Environmental Health, Part A, 2016, 79: 8-16. DOI:10.1080/15287394.2015.1099484 |
| [71] |
Wang C, Zhou LZ, Dai BG et al. The impacts of water level fluctuations between wet and dry seasons on taxonomic and functional diversity of fish communities in the ecotone floodplain of Lake Caizi. J Lake Sci, 2019, 31(5): 1403-1414. [王朝, 周立志, 戴秉国等. 水位洪枯变化对菜子湖江湖过渡带鱼类物种和功能多样性的影响. 湖泊科学, 2019, 31(5): 1403-1414. DOI:10.18307/2019.0501] |
| [72] |
Kruk A. Role of habitat degradation in determining fish distribution and abundance along the lowland Warta River, Poland. Journal of Applied Ichthyology, 2010, 23(1): 9-18. DOI:10.1111/j.1439-0426.2006.00784.x |
| [73] |
Lyubimova T, Lepikhin A, Parshakova Y et al. The risk of river pollution due to washout from contaminated floodplain water bodies during periods of high magnitude floods. Journal of Hydrology, 2016, 534: 579-589. DOI:10.1016/j.jhydrol.2016.01.030 |
 2020, Vol. 32
2020, Vol. 32 


 属Hemiculter
属Hemiculter 属Hemibarbus
属Hemibarbus