(2: 中国科学院南京地理与湖泊研究所, 南京 210008)
(2: Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing 210008, P. R. China)
沉水植物在浅水湖泊生态系统中是主要的初级生产者,有利于维持生态系统的稳定,可以改善水质,提高生物多样性[1-2].轮叶黑藻(Hydrilla verticillata)是淡水生态系统中常见的沉水植物,在农田、池塘、溪流及湖泊等淡水水体中均有分布[3].轮叶黑藻具有生长速度快、对水质改善好等特点,常被作为生态修复的先锋物种应用于湖泊修复工程[4].研究表明轮叶黑藻既可以从水中吸收营养盐,也可以从沉积物中吸收营养盐,因此其生长受水环境和沉积物环境的共同影响[5],例如,郭俊秀等[6]研究表明,当水体磷浓度低于0.2 mg/L时,轮叶黑藻的株高随着水体磷浓度增加而增加.赵强等[7]研究表明,水体磷浓度对轮叶黑藻的生长具有促进作用,尤其在水体磷浓度0.047~0.188 mg/L的范围内,随着磷浓度的增加,对轮叶黑藻的生长促进作用越强.王立志等[8]研究发现轮叶黑藻在中营养盐底质(总氮(TN)=2.71104 mg/g;总磷(TP)=0.82985 mg/g;总有机碳(TOC)=27.93598 mg/g)条件下生长状况明显好于低营养底质(TN=0.29332 mg/g; TP=0.35642 mg/g; TOC=1.24272 mg/g).
镧改性膨润土(又称锁磷剂,Phoslock®)是一种用于控制湖泊沉积物磷释放的新型材料[9].其原理是,镧改性膨润土中的5 %镧离子与沉积物中的磷酸根结合,形成难溶的絮状沉淀,即磷酸镧(LaPO4),从而改变沉积物中的磷形态,抑制沉积物磷向上覆水释放[10].大量研究表明湖泊添加锁磷剂之后,湖水中的磷浓度显著下降[11-13],同时沉积物中磷形态发生改变[9],表现为生物可利用磷(如弱结合态磷及有机磷)向生物不可用磷(如钙结合态磷)转化[14-16].目前,锁磷剂被广泛应用于欧洲温带湖泊[12]和我国热带亚热带浅水湖泊(如鄂州洋澜湖、暨南大学南湖等)的生态修复工程中,取得了积极的效果[17].
锁磷剂施用后对水体磷浓度和沉积物磷形态的改变,可能会进一步影响生长在沉积物中的沉水植物,但目前还未见报道.本研究通过中宇宙模拟实验,比较了不同水体营养盐浓度条件下,沉积物中添加锁磷剂对沉水植物轮叶黑藻生长的影响,研究结果将为湖泊修复提供科学依据.
1 材料与方法 1.1 实验设计本实验于2019年6月在中国科学院太湖湖泊生态系统研究站东太湖分站(31°03′19″N,120°42′06 E)的2个实验水池内进行,该水池长3 m、宽3 m、水深2.7 m.实验所用沉积物采自东太湖.沉积物使用前经孔径为1 cm的筛网过滤去除掉螺、贝类,然后集中放入大桶内反复搅拌,混匀备用.实验用水来自东太湖站池塘,经孔径为230 μm的筛网过滤后注入到2个实验水池中.实验所用镧改性膨润土(锁磷剂)是由澳大利亚锁磷剂环境技术有限公司生产.
实验设置2个因素:上覆水磷浓度和沉积物添加锁磷剂.每个因素2个水平,共4种处理,每种处理5个重复,共20个实验单元.每个实验单元所用的实验小桶直径24 cm、高度27 cm.首先,将充分混匀的沉积物分装到20个实验小桶中.其次,根据测定的沉积物磷形态背景值中可释放磷形态(可释放磷形态=弱结合态磷+铁锰结合态磷+有机磷)的磷酸根含量计算锁磷剂使用量[9].然后,随机挑出10个实验小桶,投放锁磷剂.投放锁磷剂时,将锁磷剂与沉积物在小桶内搅拌均匀,静置沉淀.随后,待所有实验小桶沉积物沉淀好后,向每个实验小桶内植入3株长势一致(株高15 cm)的轮叶黑藻,每个小桶轮叶黑藻的初始生物量为4.688 g/m2,再将实验小桶悬挂在水池距水面1.2 m深处.最后,向2个水池中分别添加不同浓度的营养盐来构建2种总磷(TP)浓度的上覆水,其中低浓度水池上覆水TP浓度为50 μg/L;高浓度水池上覆水TP浓度为150 μg/L.
1.2 测定指标及方法实验期间每7天采集1次水样,采样时分别在水池中选取3个点进行采集,混合均匀后装入1.5 L的矿泉水瓶中,用以测定水体中的总氮(TN)和TP浓度.我们根据TP浓度的平均值计算需要投入的营养盐,用于维持2种上覆水的低营养盐(TP=50 μg/L)和高营养盐(TP=150 μg/L)状态.每次采集水样时间为12:00—13:00,采样前先使用照度计(MQ-510)分别测定高营养盐水池及低营养盐水池距离水面0、0.2、0.4、0.6、0.8及1 m处光照强度,计算光的衰减系数,计算公式如下:
| $ K_{\mathrm{d}}=-\frac{1}{Z} \ln \frac{E(z)}{E(0)} $ | (1) |
式中,Kd为光衰减系数; z为深度; E(z)为z深度处的辐照度; E(0)为起始面辐照度[18]. TN浓度采用碱性过硫酸钾紫外分光光度法测定,TP浓度采用钼酸铵分光光度法进行测定[19-20].
实验结束时,先采集沉积物样品,测定磷形态.沉积物采样利用直径2 cm、长8 cm的圆管采集沉积物表层5 cm的沉积物柱,每个小桶采集3个不同位置的沉积物柱,混匀后放置冰箱内保存.沉积物样品磷形态分析采用煅烧法测定[21-22].磷形态采用SMT连续提取法测定[23],提取的磷形态包括弱结合态磷(NH4Cl-P)、铁锰结合态磷(BD-P)、铝结合态磷(Al-P)、钙结合态磷(Ca-P)、有机磷(Org-P)以及残渣磷(Res-P).根据文献,钙结合态磷以及残渣磷为生物不可利用磷,而弱结合态磷、铁锰结合态磷、有机磷为生物可利用磷[24-26].
实验周期42天结束后,最后将轮叶黑藻从每个实验单元中取出,在水中洗净根部底泥后,测量其株高及根长,再分别称取整株、地上部分及地下部分鲜重.轮叶黑藻相对生长率(relative growth rate, RGR)用以下公式计算:
| $ R G R=\ln \left(\frac{W_{\mathrm{f}}}{W_{\mathrm{i}}}\right) / D $ | (2) |
式中,Wi和Wf分别为植物实验前、后的鲜重,D为实验天数[27].轮叶黑藻效应量(ES)用以下公式计算[28]:
| $ E S=\ln \left(\frac{X_{1}}{X_{2}}\right) $ | (3) |
式中,X1和X2分别表示锁磷剂组生物量和无锁磷剂组生物量.
1.3 数据分析及处理实验结束时,沉积物磷形态、轮叶黑藻生物量、相对生长率及地上部分、地下部分生物量、根冠比等使用双因素方差分析(two-way ANOVA)进行比较,分析水营养盐浓度和锁磷剂的主效应和交互作用.分析之前对数据进行正态性和方差齐性检验,如不满足,则对数据进行lg转换.所有统计工作在SPSS 23.0软件中进行.
2 结果与分析 2.1 水体光照变化实验期间,低营养盐水体光衰减系数(Kd)在0.097~0.200之间波动,高营养盐水体Kd在0.104~0.228之间波动.高营养盐水体的Kd略高于低营养盐水体(图 1).

|
图 1 高营养盐水体与低营养盐水体光衰减系数变化 Fig.1 Comparison of Kd between high-nutrient water and low-nutrient water treatments |
实验结束时,在2个水体营养盐浓度条件下,较于对照(无锁磷剂),添加锁磷剂的沉积物生物可利用的铁锰结合态磷(BD-P)、铝结合态磷(Al-P)、有机磷(Org-P)含量都显著降低(P < 0.05).相反,钙结合态磷(Ca-P)含量则显著升高(P < 0.05).弱结合态磷(NH4Cl-P)含量在低营养盐水体中显著降低(P < 0.05),在高营养盐水体中则无显著性变化.残渣磷(Res-P)含量在2种营养盐环境下均没有显著性变化(P>0.05).水体营养盐浓度对沉积物P形态影响不显著(图 2).
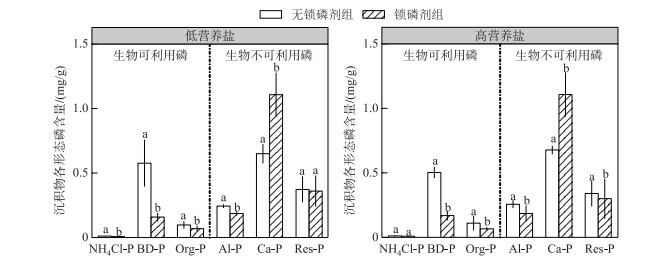
|
图 2 锁磷剂组和无锁磷剂组沉积物磷形态含量(柱状图上方的字母不同表示每种磷形态在锁磷剂组与无锁磷剂组之间的差异显著(P < 0.05) Fig.2 Phosphorus forms content of sediment under Phoslock® group and control (without Phoslock®) group (Different letters above the bars indicate statistically significant differences between Phoslock® group and control group of each phosphorus form (P < 0.05)) |
水体营养盐浓度和锁磷剂添加对轮叶黑藻生长的影响具有显著的交互作用(P < 0.05).在低营养盐水体中,无锁磷剂组轮叶黑藻生物量及相对生长率达到了锁磷剂组的2倍(P < 0.05).而在高营养盐水体中,无锁磷剂组生物量为2701.06 g/m2,略高于有锁磷剂组生物量(1999.23 g/m2),二者差异显著(P < 0.05).无锁磷剂组轮叶黑藻相对生长率为59.33 mg/(g ·d),显著高于有锁磷剂组轮叶黑藻相对生长率(48.34 mg/(g ·d))(P < 0.05)(图 3).
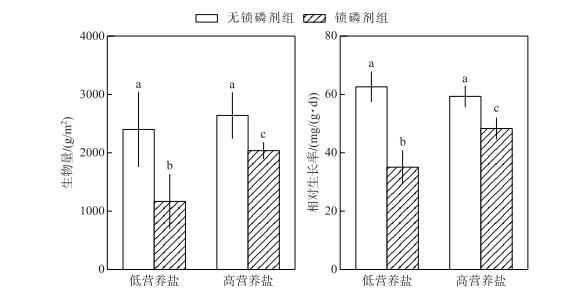
|
图 3 锁磷剂组和无锁磷剂组沉积物轮叶黑藻生物量与相对生长率(柱状图上方的字母不同表示不同处理组之间的统计学差异显著(P < 0.05)) Fig.3 Biomass and relative gross rate of sediment under Phoslock® group and control (without Phoslock®) group (Different letters above the bars indicate statistically significant differences between the different treatment groups (P < 0.05)) |
在两种营养盐水平下,沉积物添加锁磷剂均显著促进了轮叶黑藻地下部分的生长.在低营养盐水体中,锁磷剂组地下部分生物量达到456.565 g/m2,而无锁磷剂组地下部分生物量仅为82.091 g/m2,前者是后者的5倍(P < 0.05);在高营养盐水体中,锁磷剂组地下部分生物量是无锁磷剂组地下部分生物量的两倍(图 4).相反,沉积物添加锁磷剂对轮叶黑藻地上部分生长均有较明显的抑制作用.无论是高营养盐浓度,还是低营养盐浓度条件下,锁磷剂组地上部分生物量均显著低于无锁磷剂组.
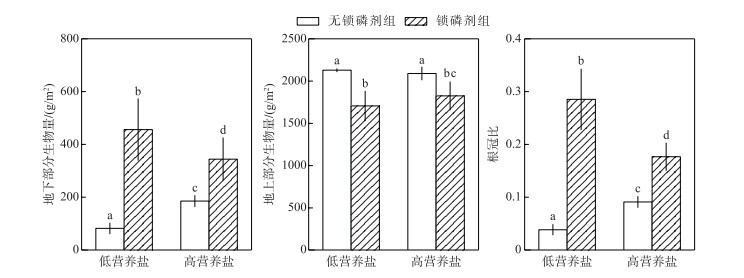
|
图 4 锁磷剂组和无锁磷剂组轮叶黑藻地下部分生物量、地上部分生物量及根冠比(柱状图上方的字母不同表示不同处理组之间的统计学差异显著(P < 0.05)) Fig.4 Aboveground biomass, underground biomass and root-shoot ratio of Hydrilla verticillata under Phoslock® group and control (without Phoslock®) group (Different letters above the bars indicate statistically significant differences between the different treatment groups(P < 0.05)) |
锁磷剂添加显著提高了轮叶黑藻的根冠比,在低营养盐水体中,锁磷剂组根冠比(0.285)是无锁磷剂组(0.038)的7倍;而在高营养盐水体中,锁磷剂组根冠比(0.176)是无锁磷剂组(0.091)的2倍.
在低营养盐水体中,锁磷剂组轮叶黑藻株高显著低于无锁磷剂组(P < 0.05),在高营养盐水体中,二者之间株高差异不显著(P>0.05).在2种不同营养盐水平下,锁磷剂组轮叶黑藻根长均显著高于无锁磷剂组(P < 0.05)(图 5).
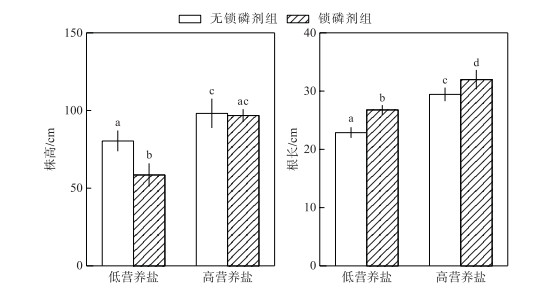
|
图 5 锁磷剂组和无锁磷剂组轮叶黑藻株高和根长(柱状图上方的字母不同表示不同组之间的统计学差异显著(P < 0.05)) Fig.5 The height and root length of Hydrilla verticillata under Phoslock® group and control (without Phoslock®) group (Different letters above the bars indicate statistically significant differences between the different treatment groups (P < 0.05)) |
在低营养盐水体中,沉积物添加锁磷剂对轮叶黑藻的生长控制的平均效应量为-0.9475,高营养盐水体中的平均效应量为-0.2520,二者差异显著(P < 0.05).
3 讨论沉积物是沉水植物生长的基质,沉水植物可以通过根系从沉积物吸收磷等营养盐用于其生长[29-30]. Verhofstad等[31]研究指出,沉水植物的生长往往随着沉积物生物可利用磷含量的增加而增加, 大量模拟实验结果也表明沉积物的养分与沉水植物的生长之间存在明显的正相关关系[32-34].例如,谢贻发研究发现,相较于营养盐较贫瘠的砂土和黏土,营养盐较丰富的湖泥更有利于沉水植物苦草的生长,其生物量、分株数量等指标均达到最高[35].镧改性膨润土是由95 %的膨润土和5 %的稀土镧组成的,膨润土的主要成分为二氧化硅,具有较大的阳离子交换量,镧离子可以较稳定地存在膨润土的结构中,并且保持较强的磷酸盐吸附能力[36]. pH值在5~9范围内,镧离子与磷酸根可以按照1 :1的比例发生反应,生成较稳定的磷酸镧沉淀,且不容易游离至水中[9].已有的研究表明,镧改性膨润土可以改变沉积物磷赋存形态. Meis等研究表明,镧改性膨润土可以使沉积物表层铁锰结合态磷转换为较稳定的钙结合态磷[9]. Lin等研究表明,沉积物添加镧改性膨润土,使得沉积物中弱结合态磷、铁锰结合态磷、铝结合态磷及有机磷含量下降,而稳定态的磷含量显著上升[37].本研究发现在2种水体营养盐浓度下,沉积物添加锁磷剂之后其生物可利用磷(铁锰结合态磷、有机磷及铝结合态磷)含量均显著降低;而生物不可用磷(如钙结合态磷)含量显著升高(图 1);锁磷剂施用后沉积物中可利用磷的减少可能是本研究中锁磷剂组轮叶黑藻生物量和相对生长率均低于无锁磷剂组的主要原因(图 3).
此外,本研究结果还表明,沉积物添加锁磷剂会改变轮叶黑藻的形态,表现为地上部分指标(如株高和地上部分生物量)降低,而地下部分指标(如根长和地下部分生物量)增加,提高轮叶黑藻的根冠比(图 4).以往的研究也发现沉水植物的形态往往会随着环境条件的变化而变化,例如李启升等[38]研究发现当水深增加,光照降低时,苦草和穗花狐尾藻均表现出地上部分生物量(株高)增加,地下部分生物量(根长)减少的形态响应,这种响应有利于植物在光照不利条件下,最大化的获取光照.本研究中,锁磷剂施用后由于沉积物可利用磷含量降低(图 2),植物通过加大对于地下部分(如根系)的生物量分配(图 4),有利于其获取更多的营养盐,维持种群生长.类似的,董百丽等[39]也发现,苦草在沉积物添加锁磷剂后有利于根系的生长,而对地上部分则表现为抑制作用.
本研究结果还表明,沉积物对轮叶黑藻生长的影响程度与水体营养盐浓度有关.在低营养盐浓度的水体中锁磷剂对植物生长和形态的影响更加显著,这可能与轮叶黑藻对营养盐的吸收方式有关.研究表明轮叶黑藻可以从沉积物和水体中吸收营养盐,取决于水体和沉积物中营养盐的可得性.唐雅丽等[40]研究发现,轮叶黑藻响应水体营养盐可得性升高时,生物量重新分配,根冠比均会降低,这样能提高植物对营养盐的吸收能力.在低营养水体中,轮叶黑藻的生长主要更加依赖于沉积物中的可利用营养盐,沉积物添加锁磷剂后可利用磷含量降低,极大了影响了轮叶黑藻的生长.然而,在高营养水体中,轮叶黑藻能够直接从水体中获取营养盐,对于沉积物可利用磷的依赖相对较低,这可能是高营养水体中轮叶黑藻对锁磷剂添加响应较低的原因.
在实施富营养型湖泊生态修复时,添加锁磷剂通常被用来控制磷的内源负荷,提高水体透明度,然后通过种植沉水植物,可以快速实现生态系统的藻-草转换,如鄂州洋澜湖、暨南大学南湖[13, 17].冠层型的轮叶黑藻繁殖速度快,对水体净化能力强.然而在修复中后期,黑藻又往往会过度繁殖,形成冠层,影响湖泊的景观和其他功能.本研究发现,通过锁磷剂的添加,降低沉积物中生物可利用磷含量,会抑制轮叶黑藻的生长,同时这种影响在水体营养盐浓度降低后更加显著.因此,在生态修复中后期适当多次投放锁磷剂,抑制内源释放、改善水质的同时,对于过度生长的轮叶黑藻具有一定的抑制作用,但在湖泊修复工程应用之前还需要进一步开展更长时间尺度和不同锁磷剂添加量的研究.
4 结论1) 沉积物添加锁磷剂改变了沉积物的磷形态,生物可利用磷含量显著降低,而生物不可用磷含量升高.
2) 2种水体营养浓度下,沉积物添加锁磷剂均显著降低了轮叶黑藻的生物量和相对生长率,同时提高了轮叶黑藻的根冠比,在低营养盐浓度条件下影响更大.
| [1] |
Carpenter SR, Lodge DM. Effects of submersed macrophytes on ecosystem processes. Aquatic Botany, 1986, 26: 341-370. DOI:10.1016/0304-3770(86)90031-8 |
| [2] |
Jeppesen E, Lauridsen TL, Kairesalo T et al. Impact of submerged macrophytes on fish-zooplankton interactions in lakes. The structuring role of submerged macrophytes in lakes. New York: Springer, 1998: 91-114. DOI: 10.1007/978-1-4612-0695-8_5.
|
| [3] |
Xu QJ, Jin XC, Wang XM et al. Effects of different concentration ammonium-N on Hydrilla verticillata antioxidant enzymes under Cd stress. Chinese Journal of Applied Ecology, 2007, 18(2): 420-424. [许秋瑾, 金相灿, 王兴民等. 不同浓度铵态氮对镉胁迫轮叶黑藻生长及抗氧化酶系统的影响. 应用生态学报, 2007, 18(2): 420-424.] |
| [4] |
Li W, Liu GH, Xiong BH. The restoration of aquatic vegetation in lakes of poyang lake nature reserve after catastrophic flooding in 1998. Journal of Wuhan Botanical Research, 2004, 22(4): 301-306. [李伟, 刘贵华, 熊秉红. 1998年特大洪水后鄱阳湖自然保护区主要湖泊水生植被的恢复. 武汉植物科学学报, 2004, 22(4): 301-306.] |
| [5] |
Liu ZW, Zhang XF, Chen FZ et al. The responses of the benthic-pelagic coupling to eutrophication and regime shifts in shallow lakes: Implication for lake restoration. J Lake Sci, 2020, 32(1): 1-10. [刘正文, 张修峰, 陈非洲等. 浅水湖泊底栖—敞水生境耦合对富营养化的响应与稳态转换机理:对湖泊修复的启示. 湖泊科学, 2020, 32(1): 1-10. DOI:10.18307/2020.0101] |
| [6] |
Guo JX, Xu QJ, Jin XC et al. Effect of phosphorus concentration on growth of Myriophyllum spicatum and Hydrilla verticillata. Acta Scientiae Circumstantiae, 2009, 29(1): 118-123. [郭俊秀, 许秋瑾, 金相灿等. 不同磷质量浓度对穗花狐尾藻和轮叶黑藻生长的影响. 环境科学学报, 2009, 29(1): 118-123.] |
| [7] |
Zhao Q. Physiological and ecological problems of Hydrilla verticillata in submerged plants in polluted water[Dissertation]. Wuhan: Institute of Hydrobiology, Chinese Academy of Sciences, 2006. [赵强.受污染水体沉水植物轮叶黑藻恢复的若干生理生态学问题研究[学位论文].武汉: 中国科学院水生生物研究所, 2006. ]
|
| [8] |
Wang LZ, Wang GX, Ge XG et al. Influence of different sediment nutrients on growth and photosynthesis fluorescence characteristics of Hydrilla verticillata (L. f) Royle. Acta Ecologica Sinica, 2010, 30(2): 473-480. [王立志, 王国祥, 葛绪广等. 底质营养盐负荷对轮叶黑藻生长和光合荧光特性的影响. 生态学报, 2010, 30(2): 473-480.] |
| [9] |
Meis S, Spears BM, Maberly SC et al. Sediment amendment with Phoslock® in Clatto Reservoir (Dundee, UK): Investigating changes in sediment elemental composition and phosphorus fractionation. Journal of Environmental Management, 2012, 93(1): 185-193. DOI:10.1016/j.jenvman.2011.09.015 |
| [10] |
Bishop WM, McNabb T, Cormican I et al. Operational evaluation of phoslock phosphorus locking technology in Laguna Niguel lake, California. Water, Air & Soil Pollution, 2014, 225(7): 2018. DOI:10.1007/s11270-014-2018-6 |
| [11] |
Robb M, Greenop B, Goss Z et al. Application of PhoslockTM, an innovative phosphorus binding clay, to two Western Australian waterways: Preliminary findings. Hydrobiologia, 2003, 494(1/2/3): 237-243. |
| [12] |
Waajen G, van Oosterhout F, Douglas G et al. Management of eutrophication in Lake De Kuil (The Netherlands) using combined flocculant—Lanthanum modified bentonite treatment. Water Research, 2016, 97: 83-95. DOI:10.1016/j.watres.2015.11.034 |
| [13] |
Gan L, Zhong P, Su L et al. Effects of Lanthanum modified bentonite on the water phosphorus concentration and sediment phosphorus form in a shallow eutrophic lake. J Lake Sci, 2019, 31(5): 1219-1228. [甘磊, 钟萍, 苏玲等. 镧改性膨润土对浅水湖泊水体磷浓度和沉积物磷形态的影响. 湖泊科学, 2019, 31(5): 1219-1228. DOI:10.18307/2019.0517] |
| [14] |
Golterman H, Paing J, Serrano L et al. Presence of and phosphate release from polyphosphates or phytate phosphate in lake sediments. Hydrobiologia, 1997, 364(1): 99-104. DOI:10.1023/A:1003212908511 |
| [15] |
Huang QH, Wang DH, Wang CX et al. Relation between phosphorus forms in the sediments and lake eutrophication. China Environmental Science, 2003, 23(6): 583-586. [黄清辉, 王东红, 王春霞等. 沉积物中磷形态与湖泊富营养化的关系. 中国环境科学, 2003, 23(6): 583-586.] |
| [16] |
Wang Q, Jiang X, Jin XC et al. Distribution of phosphorus fractions and bio-available phosphorus forms and their relationship in the sediments from different regions of Lake Taihu. J Lake Sci, 2006, 18(2): 120-126. [王琦, 姜霞, 金相灿等. 太湖不同营养水平湖区沉积物磷形态与生物可利用磷的分布及相互关系. 湖泊科学, 2006, 18(2): 120-126. DOI:10.18307/2006.0204] |
| [17] |
Wu JL, Gan L, Liu SX et al. Effect of restoration on the eutrophication and ecological status of Lake Yanglan(Hubei Province): Assessment based on macroinvertebrates. J Lake Sci, 2019, 31(6): 1547-1558. [吴家乐, 甘磊, 刘素霞等. 修复对湖北洋澜湖富营养化与生态状况的影响:基于大型无脊椎底栖动物的评价. 湖泊科学, 2019, 31(6): 1547-1558. DOI:10.18307/2019.0616] |
| [18] |
Zhang YL, Qin BQ, Chen WM et al. Analysis on distribution and variation of beam attenuation coefficient of Taihu Lake's water. Advances in Water Science, 2003, 14(4): 447-453. [张运林, 秦伯强, 陈伟民等. 太湖水体光学衰减系数的分布及其变化特征. 水科学进展, 2003, 14(4): 447-453.] |
| [19] |
American Public Health Association. American Water Works Association and Water Environment Federation. Standard methods for the examination of water and wastewater, 1998: 39-42.
|
| [20] |
Editorial Board of Water and Wastewater Monitoring and Analysis Methods, Ministry of Environmental Protection of the People's Republic of China. Water and Wastewater Monitoring and Analysis Methods: 4th edition. Beijing: China Environmental Science Press, 2002. [国家环境保护总局《水和废水监测分析方法》编委会. 水和废水监测分析方法:第4版. 北京: 中国环境科学出版社, 2002.]
|
| [21] |
Ruban V, López-Sánchez JF, Pardo P et al. Development of a harmonised phosphorus extraction procedure and certification of a sediment reference material. Journal of Environmental Monitoring, 2001, 3(1): 121-125. DOI:10.1039/b005672n |
| [22] |
Ruban V, López-Sánchez JF, Pardo P et al. Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments—A synthesis of recent works. Fresenius' Journal of Analytical Chemistry, 2001, 370(2/3): 224-228. DOI:10.1007/s002160100753 |
| [23] |
Ribeiro DC, Martins G, Nogueira R et al. Phosphorus fractionation in volcanic lake sediments (Azores-Portugal). Chemosphere, 2008, 70(7): 1256-1263. DOI:10.1016/j.chemosphere.2007.07.064 |
| [24] |
Golterman H, Paing J, Serrano L et al. Presence of and phosphate release from polyphosphates or phytate phosphate in lake sediments. Hydrobiologia, 1997, 364(1): 99-104. DOI:10.1023/A:1003212908511 |
| [25] |
Huang QH, Wang DH, Wang CX et al. Relation between phosphorus forms in the sediments and lake eutrophication. China Environmental Science, 2003, 23(6): 583-586. [黄清辉, 王东红, 王春霞等. 沉积物中磷形态与湖泊富营养化的关系. 中国环境科学, 2003, 23(6): 583-586.] |
| [26] |
Wang Q, Jiang X, Jin XC et al. Distribution of phosphorus fractions and bio-available phosphorus forms and their relationship in the sediments from different regions of Lake Taihu. J Lake Sci, 2006, 18(2): 120-126. [王琦, 姜霞, 金相灿等. 太湖不同营养水平湖区沉积物磷形态与生物可利用磷的分布及相互关系. 湖泊科学, 2006, 18(2): 120-126. DOI:10.18307/2006.0204] |
| [27] |
Li KY, Liu ZW, Li CH et al. The herbivory effects of snail Radix swinhoei on submerged plant community. Oceanologia et Limnologia Sinica, 2007, 38(6): 576-580. [李宽意, 刘正文, 李传红等. 螺类牧食损害对沉水植物群落结构的调节. 海洋与湖沼, 2007, 38(6): 576-580.] |
| [28] |
Hoekman D. Turning up the heat: Temperature influences the relative importance of top-down and bottom-up effects. Ecology, 2010, 91(10): 2819-2825. DOI:10.1890/10-0260.1 |
| [29] |
Rattray MR, Howard-Williams C, Brown JMA. Sediment and water as sources of nitrogen and phosphorus for submerged rooted aquatic macrophytes. Aquatic Botany, 1991, 40(3): 225-237. DOI:10.1016/0304-3770(91)90060-i |
| [30] |
Xie YH, An SQ, Yao X et al. Short-time response in root morphology of Vallisneria natans to sediment type and water-column nutrient. Aquatic Botany, 2005, 81(1): 85-96. DOI:10.1016/j.aquabot.2004.12.001 |
| [31] |
Verhofstad MJJM, Alirangues NMM, Reichman EP et al. Mass development of monospecific submerged macrophyte vegetation after the restoration of shallow lakes: Roles of light, sediment nutrient levels, and propagule density. Aquatic Botany, 2017, 141: 29-38. DOI:10.1016/j.aquabot.2017.04.004 |
| [32] |
Barko JW, Smart RM. Sediment-related mechanisms of growth limitation in submersed macrophytes. Ecology, 1986, 67(5): 1328-1340. DOI:10.2307/1938689 |
| [33] |
Martin GD, Coetzee JA. Competition between two aquatic macrophytes, Lagarosiphon major (Ridley) moss (Hydrocharitaceae) and Myriophyllum spicatum Linnaeus (Haloragaceae) as influenced by substrate sediment and nutrients. Aquatic Botany, 2014, 114: 1-11. DOI:10.1016/j.aquabot.2013.11.001 |
| [34] |
Angelstein S, Wolfram C, Rahn K et al. The influence of different sediment nutrient contents on growth and competition of Elodea nuttallii and Myriophyllum spicatum in nutrient-poor waters. Fundamental and Applied Limnology, 2009, 175(1): 49-57. DOI:10.1127/1863-9135/2009/0175-0049 |
| [35] |
Xie YF. Study on interaetion of submerged macrophytes and nutrients in the water and sediment[Dissertation]. Guangzhou: Jinan University, 2008. [谢贻发.沉水植物与富营养湖泊水体、沉积物营养盐的相互作用研究[学位论文].广州: 暨南大学, 2008. ]
|
| [36] |
Copetti D, Finsterle K, Marziali L et al. Eutrophication management in surface waters using lanthanum modified bentonite: A review. Water Research, 2016, 97: 162-174. DOI:10.1016/j.watres.2015.11.056 |
| [37] |
Lin J, Qiu PH, Yan XJ et al. Effectiveness and mode of action of calcium nitrate and Phoslock® in phosphorus control in contaminated sediment, a microcosm study. Water, Air, & Soil Pollution, 2015, 226(10): 330. DOI:10.1007/s11270-015-2590-4 |
| [38] |
Li QS, Huang Q, Li YJ et al. Effects of water depth on growth of submerged macrophytes Vallisneria natans and Myriophyllum spicatum. J Lake Sci, 2019, 31(4): 1045-1054. [李启升, 黄强, 李永吉等. 水深对沉水植物苦草(Vallisneria natans)和穗花狐尾藻(Myriophyllum spicatum)生长的影响. 湖泊科学, 2019, 31(4): 1045-1054. DOI:10.18307/2019.0404] |
| [39] |
Dong BL, Qin BQ, Gong ZJ et al. Effects of sediment amendment on Vallisneria natans growth. Chinese Journal of Ecology, 2011, 30(12): 2726-2731. [董百丽, 秦伯强, 龚志军等. 三种沉积物改良措施比较及其对苦草生长的影响. 生态学杂志, 2011, 30(12): 2726-2731.] |
| [40] |
Tang YL, Fu BZ, Zhang XF et al. Nutrient addition delivers growth advantage to Hydrilla verticillata over Vallisneria natans: A mesocosm study. Knowledge & Management of Aquatic Ecosystems, 2019, 73(420): 12. DOI:10.1051/kmae/2018046 |
 2021, Vol. 33
2021, Vol. 33 

